Abstract
PURPOSE Potentially inappropriate prescribing (PIP) is a common yet preventable medical error among older persons in primary care. It is uncertain whether PIP produces adverse outcomes in this population, however. We conducted a systematic review with meta-analysis to pool the adverse outcomes of PIP specific to primary care.
METHOD We searched PubMed, Embase, CINAHL, Web of Science, Scopus, PsycINFO, and previous review articles for studies related to “older persons,” “primary care,” and “inappropriate prescribing.” Two reviewers selected eligible articles, extracted data, and evaluated the risk of bias. Meta-analysis was conducted to pool studies with similar PIP criteria and outcome measures.
RESULTS Of the 2,804 articles identified, we included 8 articles with a total of 77,624 participants. All included studies had cohort design and low risk of bias. Although PIP did not affect mortality (risk ratio [RR] 0.98; 95% CI, 0.93-1.05), it was significantly associated with the other available outcomes, including emergency room visits (RR 1.63; 95% CI, 1.32-2.00), adverse drug events (RR 1.34; 95% CI, 1.09-1.66), functional decline (RR 1.53; 95% CI, 1.08-2.18), health-related quality of life (standardized mean difference –0.26; 95% CI, –0.36 to –0.16), and hospitalizations (RR 1.25; 95% CI, 1.09-1.44). A majority of the pooled estimates had negligible heterogeneity.
CONCLUSIONS This meta-analysis provides consolidated evidence on the wide-ranging impact of PIP among older persons in primary care. It highlights the need to identify PIP in primary care, calls for further research on PIP interventions in primary care, and points to the need to consider potential implications when deciding on the operational criteria of PIP.
- primary health care
- general practice
- general practitioners
- family practice
- family physicians
- inappropriate prescribing
- medication errors
- aged
- adverse outcomes
- systematic review
- meta-analysis
INTRODUCTION
Potentially inappropriate prescribing (PIP) is the prescribing, or underprescribing, of medications for older persons that may cause significant harm.1 It can be evaluated by holistic criteria to assess older persons’ prescriptions in the context of multiple comorbidities, complex medication regimes, functional and cognitive status, treatment goals, and life expectancy.1 In the literature, it has been often been operationalized with simpler screening criteria that can be further classified as implicit (judgement based) or explicit (criterion based).1 Implicit (judgment based) tools are quality indicators that clinicians can apply to a prescription to judge the prescribing appropriateness, and they include the Medication Appropriateness Index. They can often be time consuming to use and rely on each clinician’s medical knowledge, which limits their use in clinical practice.1 Explicit (criterion based) tools comprise lists of drugs or drug classes (developed from literature reviews, expert opinion, and consensus techniques) that when prescribed or underprescribed can cause harm in older persons. They have been more widely adopted in the literature2 because of the simplicity of their administration, and they include tools such as the Beers Criteria for Potentially Inappropriate Medication Use in Older Adults (Beers Criteria), Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP), and Screening Tool to Alert Doctors to Right Treatment (START).1–3
In the literature, the pooled prevalence of PIP ranges from 22.6% for community-dwelling older persons4 to 43.2% for nursing home residents.5 Previous reviews3,6,7 have highlighted the associations of PIP with adverse drug events (ADEs), lower quality of life, hospitalizations, and higher health care costs. There has also been 1 meta-analysis8 that demonstrated the significant effects of PIP on mortality (risk ratio [RR] 1.59; 95% CI, 1.45-1.75). The relationship between PIP and adverse outcomes may be related to the changes in pharmacokinetics and pharmacodynamics in older persons, especially among those who are frail, where there is limited physiologic reserve and lower tolerance of inappropriate medication use.3
With rapid aging of populations across the world,9 we can expect an increasing number of older persons who have chronic diseases and need regular prescriptions from primary care. Although a recent systematic review reports the prevalence of PIP as 1 in 5 older persons attending primary care,10 it remains unclear whether such PIP produces adverse outcomes in this population. Previous reviews3,6–8 have included a heterogeneous range of participants, with much of the focus on specialized populations such as those from tertiary health care settings or nursing homes. We are uncertain whether the results from tertiary health care settings or nursing homes can be generalized to primary health care settings, considering that the patients in primary care can differ in their health profiles and comorbidities.11,12 To this end, we sought to conduct a systematic review with meta-analysis to pool the adverse outcomes of PIP reported in the literature, specifically focusing on older persons in primary care.
METHODS
Search Strategy and Selection Criteria
This systematic review and meta-analysis was developed in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis statement.13 The protocol was registered with the International Prospective Register of Systematic Reviews (registration number CRD42016048874) and previously peer reviewed and published in BMJ Open.14
We systematically searched PubMed, Embase, CINAHL, Web of Science, Scopus, and PsycINFO from inception to January 7, 2017, using keywords and controlled vocabulary related to “older persons,” “primary care,” and “inappropriate prescribing.” A sample of the search strategy based on PubMed is shown in the Supplemental Appendix, available at http://www.AnnFamMed.org/content/17/3/257/suppl/DC1/.
Similar search strategies were used for the other databases. We also hand searched the references of 6 review articles related to PIP3,4,6–8,10 to retrieve relevant articles that were not captured through our search of the electronic databases. Included studies met the following criteria: recruited participants from primary care settings; either had ≥90% of the participants who were aged ≥65 years or reported subgroup analyses based on participants who were aged ≥65 years; were conducted via observational study designs, such as cross-sectional, case-control, or cohort studies; reported the adverse outcomes related to PIP, such as accident and emergency department (A&E) visits, ADEs, functional decline, health-related quality of life (HRQoL), hospitalizations, and mortality; and were reported in the English language. Studies were excluded if they recruited participants from non–primary care settings, such as tertiary hospitals or nursing homes; did not assess PIP based on published criteria; or focused only on PIP related to a single class of drug, such as analgesics or antibiotics.
After retrieving potential articles, 2 reviewers (S.K.L.G., Z.Y.C.) independently selected eligible articles, extracted the relevant data, and assessed the risk of bias. Any discordance between the 2 reviewers was resolved by discussion with a third independent reviewer (C.S.L. or T.M.L.). The extracted data included information on participants, study characteristics, criteria of PIP, measurement of adverse outcomes, effect estimates, and their 95% CI. The risk of bias was assessed with the original 8-item Newcastle-Ottawa scale,15 which focuses on 3 key areas of potential bias: selection of participants, comparability of groups, and measurement of outcome. The exact items in the Newcastle-Ottawa scale are shown in the Supplemental Appendix.
Data Analysis
We conducted meta-analyses to pool the results for available outcomes that had >1 included study. We used the fixed-effect model (Mantel-Haenszel method)16 in the meta-analysis because the random effects model would have been imprecise in its estimations when <5 studies were included in the analysis.17,18 For studies that reported >1 model of statistical adjustment, we included only the result with the largest number of confounders adjusted for in the statistical model. For continuous outcomes, we pooled the results based on Cohen’s standardized mean difference (SMD). For binary outcomes, we log-transformed the effect estimates before including them in the meta-analytic models. Although the RR and odds ratio have been used in different studies, we chose RR as a measure of risk estimates in our meta-analyses because the odds ratio would have approximated the RR in the presence of low incidence rate of the outcomes.19 We used the Q test and the I2 statistic to assess the extent of het erogeneity among the pooled studies, with Q test P value <.10 or I2 >75% indicating significantly high heterogeneity.20 To minimize heterogeneity, we pooled only studies with similar PIP criteria and outcome measures. Although originally planned, we were not able to conduct meta-regression analysis or evaluate for publication bias because these analyses would not have been appropriate when there are <10 included studies.21 All analyses were conducted in Stata version 14 (StataCorp LP).
We used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) framework to classify the overall certainty of evidence into 1 of 4 levels (high, moderate, low, or very low) based on 7 key domains that focused on risk of bias, inconsistency, imprecision of effect estimates, risk of publication bias, indirectness of evidence, large magnitude of effect, and dose-response gradient.22 Detailed descriptions of the other domains in GRADE are shown in the Supplemental Appendix.
RESULTS
We identified 2,804 articles from the 6 databases. After the selection process, we included 8 articles that are related to 6 unique studies (2 pairs of articles23–26 reported different outcome measures originating from the same studies). The flowchart with details of the selection process is shown in Figure 1. The 6 included studies had a total of 77,624 participants. All of them were cohort studies, with a mean follow-up duration of 2.0 years. The key characteristics of the included studies are presented in Table 1 All the included studies had low risk of bias and achieved maximum or near-maximum scores on the Newcastle-Ottawa Scale (the summary results are shown in Table 1, and the detailed results are shown in the Supplemental Appendix. All included studies reported results based on adjusted estimates. The covariates that were adjusted for in the statistical models are presented in Table 2. They include key confounders such as age, socioeconomic status, disease comorbidities, and number of medications.
Flowchart of the study selection.
Key Characteristics of the Studies Included in the Systematic Review
Covariates That Were Adjusted for in the Statistical Models of the Included Studies
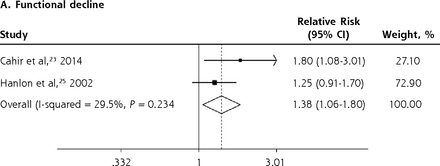
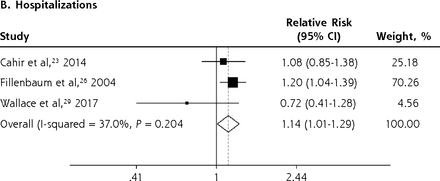
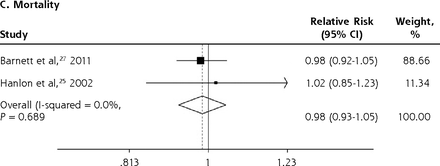
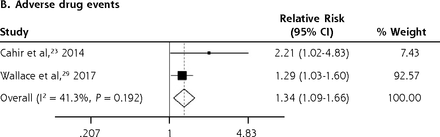
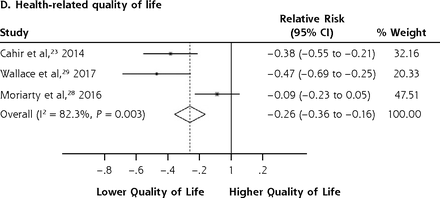
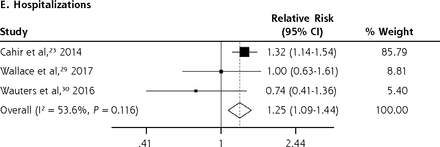
We conducted meta-analyses only when there were ≥2 studies with results based on similar PIP criteria and outcome measures; results that could not be pooled in the meta-analysis are separately shown in the Supplemental Appendix. The PIP criteria commonly used in the included studies were the Beers Criteria and the STOPP criteria. Six outcome measures were consistently reported in ≥2 studies and could be subjected to meta-analysis, including A&E visits, ADEs, functional decline, HRQoL, hospitalizations, and mortality. Figure 2 shows the forest plots for the adverse outcomes of PIP based on the Beers Criteria, with PIP significantly associated with functional decline (pooled RR 1.38; 95% CI, 1.06-1.80) and hospitalizations (pooled RR 1.14; 95% CI, 1.01-1.29) but not with mortality (pooled RR 0.98; 95% CI, 0.93-1.05). Figure 3 shows the forest plots for the adverse outcomes of PIP based on the STOPP criteria, with PIP significantly associated with A&E visits (pooled RR 1.63; 95% CI, 1.32-2.00), ADEs (pooled RR 1.34; 95% CI, 1.09-1.66), functional decline (pooled RR 1.53; 95% CI, 1.08-2.18), HRQoL (pooled SMD –0.26; 95% CI, –0.36 to –0.16), and hospitalizations (pooled RR 1.25; 95% CI, 1.09-1.44).
Forest plots for the adverse outcomes of potentially inappropriate prescribing based on the Beers Criteria.
Forest plots for the adverse outcomes of potentially inappropriate prescribing based on the STOPP (Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions) criteria.
A&E = accident and emergency department; HRQoL = health-related quality of life.
A majority of the pooled estimates had negligible heterogeneity. Notwithstanding that, 1 of the outcomes, HRQoL, showed significantly high heterogeneity (I2 82.3% and P = 0.003) because of the different scales used in measuring HRQoL (2 studies24,29 used the EQ-5D, and 1 study28 used the Control, Autonomy, Self-realization, and Pleasure Revised 12-item Quality of Life scale). The GRADE assessment of overall certainty of evidence is shown in Table 3. Apart from HRQoL, the rest of the outcomes maintained “low” certainty of evidence consistent with meta-analytic results based on observational studies, with no further downgrades or upgrades in the GRADE assessment. HRQoL was downgraded to “very low” certainty of evidence because of the high heterogeneity.
Summary of Key Findings and GRADE Assessment
DISCUSSION
This is the first systematic review and meta-analysis of observational studies on the adverse outcomes of PIP among older persons in primary care. Although PIP is not associated with mortality in this study, it is significantly associated with the other available outcomes, including A&E visits, ADEs, functional decline, HRQoL, and hospitalizations. The meta-analytic results were based on cohort studies with large numbers of participants, samples representative of the primary health care settings, and low risk of bias. Moreover, most of the pooled estimates had low heterogeneity, which suggests consistency of results across the included studies.
Comparison With Other Studies
To date, only 1 other meta-analysis has reported on the adverse outcomes of PIP.8 This meta-analysis by Muhlack et al8 included a heterogeneous range of populations (with a large proportion of participants from tertiary health care settings or nursing homes) and focused mainly on the outcome of mortality, however. Our result on mortality showed both similarity and dissimilarity to that of Muhlack et al.8 In the current meta-analysis, we found that PIP (assessed at a single time point, also known as the prevalent-user design) is not associated with mortality longitudinally (pooled RR 0.98; 95% CI, 0.93-1.05). This is similar to the result by Muhlack et al,8 where the pooled RR based on prevalent-user design of PIP is 1.01 (95% CI, 0.97-1.04). However, Muhlack et al8 also identified a subset of studies that evaluated PIP based on the new-user design (which also captures those with new onset of PIP) and showed that the risk of mortality is higher (RR 1.59; 95% CI, 1.45-1.75) when PIP is based on evaluations at multiple time points. The discrepancy in findings is consistent with our understanding of the prevalent-user design and its association with healthy-user/sick-stopper bias, which can underestimate the true effect.8,31,32 At any specific time point, those found to have PIP according to the prevalent-user design are more likely to be healthier people who have better physiologic reserve and can adhere to the treatment regimen even in the presence of PIP. They may be less affected by PIP and are less likely to die from PIP. In contrast, those who have poorer health would be more likely to have discontinued PIP because of poor tolerance and may not be captured by the prevalent-user design at a specific time point. Although these people may have a higher risk of dying from PIP, the exclusion of them in a prevalent-user design can bias the result toward null effect, which may possibly explain the nonsignificant finding on mortality in the current meta-analysis.
Limitations
Several limitations should be noted before the implications of the current findings are considered. First, we included only studies reported in the English language and may have missed other relevant evidence in the non-English literature. Second, the limited number of included studies did not allow further investigations of individual- or study-level associations. Third, we did not include studies of PIP that are specific to certain drug classes (eg, analgesics or antibiotics) and therefore may not draw conclusions about the outcomes associated with specific classes of drugs. Fourth, although we also identified studies of PIP related to underprescribing (eg, those using the START criteria), we could not pool results in the meta-analysis because of the limited number of available studies related to underprescribing (Supplementary Appendix), which remains an area for further research in future. Fifth, there were some variations in the magnitude of pooled estimates between the Beers and STOPP criteria, which were probably related to the different drugs included in the respective criteria (some of which do not necessarily apply to the different populations).1 Sixth, the meta-analytic results were based on observational studies, which may not have sufficiently controlled for all relevant confounders. Therefore, the findings may not allow definitive conclusions about the causal role between PIP and the adverse outcomes.
Clinical Implications
This study demonstrated the associations between PIP and a wide range of adverse outcomes and highlighted the relevance of PIP among older persons in primary care. Essentially, the findings showed that the construct of PIP is more than just a consensus of good clinical practice, and they underscored the need to focus on PIP in primary care to improve patient outcomes. A variety of interventions have been evaluated to address PIP among community-dwelling older persons in previous randomized controlled trials (RCTs), some of which may be adopted in routine practice. These interventions can be broadly classified into organizational, professional, or multifaceted interventions.33 Organizational interventions focus on changing the delivery of health care services (eg, by including medication reviews by pharmacists), professional interventions aim to improve the practice of health care professionals (eg, with the use of computerized clinical decision support systems [CCDSSs]), and multifaceted interventions may involve combinations of organizational or professional interventions. Most of the interventions have been shown to be useful in the literature, with some of them (eg, medication reviews, CCDSSs, and multifaceted interventions) having had consistent evidence of effectiveness in reducing PIP.33
Implications for Future Research
Notwithstanding the promising benefits of interventions, more research is needed to evaluate the effects of interventions on clinically relevant outcomes. Prior RCTs have infrequently reported on the clinical outcomes of interventions, and the few that have done so had mixed results for HRQoL, hospitalizations, A&E visits, and medication costs.33 Such research is especially relevant in light of the current meta-analytic findings, to further confirm that the reduction of PIP can have a direct effect in improving outcomes and that PIP has a direct causal relationship with the adverse outcomes. Ideally, future RCTs should be adequately powered and have a sufficiently long follow-up period so that any difference in the outcomes can be captured sensitively.33 Inasmuch as intervention studies are relevant, further research is also needed in the area of implementation science, to evaluate how PIP interventions can be optimally integrated into routine clinical practice, especially in primary health care settings where the patient load can be high and changes can be difficult to implement or sustain.
In conducting future studies, researchers should be mindful of how PIP is operationalized and of the potential implications associated with the choice of PIP criteria. Researchers may consider capturing PIP based on the new-user design (which includes those with new onset of PIP)—instead of the conventional approach of PIP based on prevalent-user design (which identifies those with PIP only at a single time point)—to improve the predictive validity of PIP and to identify patients who can receive a greater benefit from PIP interventions. When selecting a screening tool for PIP, researchers may need to consider the applicability of individual drugs within the tool and possibly adapt the tool to fit the local prescribing practice. Researchers may also need to be aware that different tools may capture different aspects of PIP. Although most available tools focus on overprescribing or misprescribing of medications, it may be equally important to include tools related to underprescribing of medications (such as the START) to capture the whole spectrum of PIP.
CONCLUSION
This is the first meta-analysis to consolidate the quantitative evidence on the wide-ranging impact of PIP among older persons in primary care. The outcome of mortality was the only nonsignificant finding, possibly explained by the recruitment of healthier participants, which may have biased the results toward null effect. The findings highlight the need to address PIP in primary care and call for further research on PIP interventions in primary care. They also point to the need for researchers to consider the potential implications of how PIP is operationalized when designing future research on PIP.
Footnotes
↵* These authors contributed equally to this work.
Conflicts of interest: authors report none.
To read or post commentaries in response to this article, see it online at http://www.AnnFamMed.org/content/17/3/257.
Author contributions: All authors have read and approved the manuscript. Study concept: T.M.L., C.S.L. Review of the literature: C.S.L. Study methodology and search strategy: T.M.L. Article selection: S.K.L.G., Z.Y.C. Data extraction: S.K.L.G., Z.Y.C., C.S.L. Analysis of data: T.M.L. Interpretation of data: T.M.L., C.S.L. Drafting of the manuscript: T.M.L. Revision of the manuscript: T.M.L., C.S.L., S.K.L.G. Formatting of the manuscript: C.S.L. Project supervision: T.M.L., C.S.L.
Funding support: T.M.L. was supported by research grants under the Singapore Ministry of Health’s National Medical Research Council (grant nos. NMRC/Fellowship/0030/2016 and NMRC/CSSSP/0014/2017). The funding sources had no involvement in any part of the project.
Supplementary materials: Available at http://www.AnnFamMed.org/content/17/3/257/suppl/DC1/.
- Received for publication July 14, 2018.
- Revision received January 15, 2019.
- Accepted for publication January 30, 2019.
- © 2019 Annals of Family Medicine, Inc.