Abstract
PURPOSE Although cesarean delivery is the most common surgical procedure in the United States, postoperative opioid prescribing varies greatly. We hypothesized that patient characteristics, procedural characteristics, or both would be associated with high vs low opioid use after discharge. This information could help individualize prescriptions.
METHODS In this prospective cohort study, we quantified opioid use for 4 weeks following hospital discharge after cesarean delivery. Predischarge characteristics were obtained from health records, and patients self-reported total opioid use postdischarge on weekly questionnaires. Opioid use was quantified in milligram morphine equivalents (MMEs). Binomial and Poisson regression analyses were performed to assess predictors of opioid use after discharge.
RESULTS Of the 233 patients starting the study, 203 (87.1%) completed at least 1 questionnaire and were included in analyses (86.3% completed all 4 questionnaires). A total of 113 patients were high users (>75 MMEs) and 90 patients were low users (≤75 MMEs) of opioids postdischarge. The group reporting low opioid use received on average 44% fewer opioids in the 24 hours before discharge compared with the group reporting high opioid use (mean = 33.0 vs 59.3 MMEs, P <.001). Only a minority of patients (11.4% to 15.8%) stored leftover opioids in a locked location, and just 31 patients disposed of leftover opioids.
CONCLUSIONS Knowledge of predischarge opioid use can be useful as a tool to inform individualized opioid prescriptions, help optimize nonopioid analgesia, and reduce opioid use. Additional studies are needed to evaluate the impact of implementing such measures on prescribing practices, pain, and functional outcomes.
- pain
- postoperative
- pain management
- analgesia
- obstetrical
- cesarean section
- opioids
- controlled substances
- analgesics
- non-narcotic
- practice-based research
INTRODUCTION
Family medicine physicians direct both in-hospital and postdischarge care of women undergoing cesarean delivery.1 This is the most common surgical procedure performed in the United States, with 1.23 million performed in 2017.2 Most patients who have a cesarean delivery receive a prescription for opioids on discharge from the hospital.3 Although persistent opioid use after cesarean delivery is rare, overprescribing creates a pool of uncontrolled opioids in the community, which poses a potential risk for nonmedical use.4–6 In the United States, 53% of people who abuse prescription opioids report getting their last pill from a friend or family member.7 Overprescribing poses an additional danger for patients undergoing cesarean delivery because of the presence of young children in the home. It has been shown that up to 77% of individuals store their opioid medications in unlocked locations, and nearly all opioid exposures in children are from medications prescribed to an adult in the household.8–10 One study found that young children of mothers prescribed opioids have a 2.4-fold increased risk for opioid overdose.11 These statistics demonstrate the grave danger of having excess, unlocked opioid pills in a household with young children.
Meanwhile, there is a delicate balance between limiting excess opioid medications and providing adequate analgesia to patients. Undertreatment of pain in patients undergoing cesarean delivery has been associated with an increased risk of chronic pain, postpartum depression, negative impacts on infant care, and difficulty breastfeeding.12–14 Currently, limited data exist to guide discharge opioid prescribing for postcesarean patients, resulting in marked variability in prescribing practices. In 2 recent studies, the total milligram morphine equivalents (MMEs) prescribed at discharge after cesarean delivery ranged from 25 to 1,950 MMEs, or 3.3 to 260 oxycodone 5-mg tablets.15,16 It has also been shown that discharge opioid prescriptions do not correlate with 24-hour predischarge opioid use or predischarge pain scores.17,18 In general, there is a lack of consensus regarding oral analgesic regimens for patients who have undergone cesarean delivery.19 To mitigate these risks, evidence-based guidelines are needed to help clinicians determine the appropriate opioid prescription for each patient.
Our study sought to define patient and procedural characteristics that are associated with high vs low opioid use postdischarge after a cesarean delivery. According to expert suggestions,20 we chose as the upper limit for low opioid use 75 MMEs, or 10 oxycodone 5-mg tablets. Information likely to predict high vs low use could help reduce both overprescribing and undertreatment by identifying patient opioid needs. Such a tailored approach could allow for more responsible prescribing of opioid medications, in turn reducing the potential harm from excess pills in the community. A secondary aim of this study was to describe postdischarge use of nonopioid analgesics: nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen are widely accepted as part of a balanced analgesic regimen after cesarean delivery.21,22 These medications are frequently optimized in the inpatient setting, partly because of their inclusion in standardized postoperative order sets. Evidence-based guidelines for postcesarean opioid prescribing should also include recommendations for optimizing nonopioid analgesics after discharge to minimize potentially high risk opioid prescribing.23
METHODS
This prospective cohort study was approved by the Colorado Multiple Institutional Review Board under protocol 14-1938. We developed a questionnaire to quantify postoperative opioid use during the first 4 weeks following hospital discharge after cesarean delivery. The questionnaire included questions from the National Institutes of Health Patient-Reported Outcomes Measurement Information System (PROMIS) tool to evaluate pain severity and pain interference after discharge.24 Item inclusion and wording were informed by our multidisciplinary research group, and readability was assessed using the Flesch-Kincaid grade level score.25 The questionnaire was subsequently tested for face validity among 61 patients.8 Our study is presented here in concordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for reporting observational studies.26
Patient Enrollment
Female inpatients who underwent cesarean delivery at the University of Colorado Hospital between February 2, 2017 and April 2, 2018 were screened for eligibility using the electronic health record. Exclusion criteria were an inability to understand English or Spanish, infectious precautions orders that limited our access for enrollment, incarceration, planned discharge to a long-term hospital or treatment program, and inability to be contacted after discharge (eg, because of homelessness). Eligible patients were compiled into a spreadsheet, and their order of contact was randomized using a digital randomization algorithm. According to this order, patients were met individually in the hospital by a member of our research group. Access to the questionnaire was enhanced in October 2017 by implementing a research protocol amendment to permit engagement of medical translators to introduce the Spanish-language questionnaire. Nine Spanish-speaking patients were enrolled after this change. Patients were given a 2-page introductory letter outlining the study. Informed consent was given by patients when answering the questionnaire as specified by the Colorado Multiple Institutional Review Board protocol. Participation was completely voluntary. A gift card for $10 was offered for completion of each weekly questionnaire, totaling $40 available to each participant.
Data Collection
We collected predischarge data from the electronic health record, including patient demographics, preoperative opioid use, American Society of Anesthesiology (ASA) physical status, type and duration of procedure, associated procedures, opioid and nonopioid analgesics used in the 24 hours before discharge, and the discharge opioid prescription type and amount. ASA physical status classes are defined as follows: I, normal healthy patient; II, patient with mild systemic disease; III, patient with severe systemic disease; IV, patient with severe systemic disease that is a constant threat to life; V, moribund patient who is not expected to survive without the operation; and VI, cadaveric organ donor.27 The questionnaire was made available online, by telephone, or by mailed paper copy, and was available in both English and Spanish languages, depending on participant preference. Starting at 1 week postdischarge, participants received once-weekly questionnaires for 4 weeks that they returned by the same method used for access. Survey data were collected using Research Electronic Data Capture (Vanderbilt University), a secure web application designed to support data capture for research studies.28
Outcomes
The primary outcome of this study was the total patient-reported MMEs taken over the first 4 weeks postdischarge after cesarean delivery. We calculated MMEs using knowledge of drug type, route of administration, and commonly accepted conversion factors.29 Total opioid use over the 4 weeks postdischarge was dichotomized into high use (>75 MMEs) and low use (≤75 MMEs) categories, with 75 MME representing the equivalent of 10 oxycodone 5-mg tablets. We chose this cutoff primarily according to suggestions by experts,20 who recommend 75 MMEs or 10 oxycodone 5-mg tablets as the upper limit for opioid prescriptions following discharge after cesarean delivery. This cutoff divided the cohort into 2 roughly similar-sized groups. Secondary outcomes included the amount of unused opioids, postdischarge use of nonopioid analgesics such as over-the-counter acetaminophen and NSAIDs, PROMIS scores for postdischarge pain, reasons for not taking any opioids, and opioid storage and disposal practices. We also ascertained from the electronic health record whether patients received additional opioid prescriptions on days 1 through 28 after discharge.
Statistical Analysis
We hypothesized that patient characteristics, procedural characteristics, or both would be associated with high vs low self-reported opioid use after discharge. Groups were characterized using descriptive measures, including percentages, means, medians, and standard deviations. Separate binomial regression analyses were first performed for all predischarge factors to assess associations between high vs low opioid use. For variables with questionable convergence, a modified Poisson regression approach was used.30 We evaluated associations between level of use and postoperative patient-reported outcomes over the 4 weeks following discharge with separate binomial regression analyses or modified Poisson regression analyses accounting for repeated measures on participants as indicated.31,32 Significant group-by-week interactions were followed by post hoc comparisons of groups at each week.
We next attempted multiple binomial regression analysis to jointly evaluate the preoperative variables that separately were significant predictors. Because the lack of model convergence precluded any comparisons using this approach, the modified Poisson regression approach was again used to accurately estimate adjusted risk ratios for predicting high vs low opioid use after hospital discharge.30 Analyses for significant preoperative variables were repeated in the subset of patients who had complete data and in the subset of patients who reported no preoperative opioid use. Statistical comparisons were made using SAS v9.4 (SAS Institute, Inc).
RESULTS
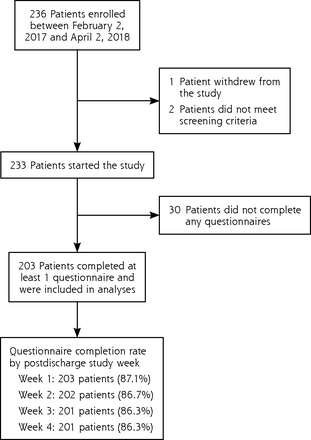
Overall, 203 patients completed at least 1 questionnaire and were included in analyses. Study enrollment and follow-up are summarized in Figure 1.
Flow chart of study enrollment and follow-up.
On the basis of total self-reported MMEs taken over the first 4 weeks postdischarge, 113 patients fell into the high opioid use group (>75 MMEs) and 90 patients into the low opioid use group (≤75 MMEs). Patient, procedural, and perioperative characteristics for both groups are shown in Table 1. Only 3 baseline patient characteristics differed significantly. More patients in the high use group (5 patients) compared with the low use group (zero patients) used opioids preoperatively. Also, compared with the low use group, the high use group used more MMEs and were more likely to receive acetaminophen in the 24 hours before discharge. Patients in both groups received the same median prescription of 225 MMEs at discharge.
Comparison of Patient, Procedural, and Perioperative Characteristics by Self-Reported Postdischarge Opioid Use (N = 203)
Table 2 shows the comparison of postdischarge patient-reported outcomes regarding opioid and nonopioid intake as well as pain by postdischarge opioid use. The amount of opioids reportedly taken decreased over the 4 weeks postdischarge, with the median being 0 MMEs in the low use group during weeks 2 through 4, and in the high use group in weeks 3 and 4. Accordingly, PROMIS scores for pain intensity and interference both decreased as well. When comparing outcomes between the low and high use groups over time, only NSAID intake was significantly higher in weeks 2 through 4 in the group with high opioid use. Of the 203 patients, 20 (10%) received opioid prescriptions in addition to their original discharge opioid prescription.
Comparison of Patient-Reported Use of Opioids and OTC Analgesics, and Pain Over Time
Patients’ reasons for not taking opioids after discharge as well as storage and disposal practices are outlined in Table 3. The most common reason for not taking opioids was adequate pain control without them, followed by concerns about breastfeeding. Only 11% to 16% of patients reported storing leftover opioids in a locked location, and merely 3% to 5% of patients reported disposing of leftover opioids. Just 31 patients (15.3%) disposed of leftover opioids.
Patient-Reported Leftover Opioids and Storage and Disposal
Table 4 shows the results of the multiple Poisson regression models incorporating significant variables from Table 1 to estimate adjusted risk ratios for predicting high vs low opioid use. Results were similar for the full sample of 203 patients, for the subsample of 201 patients completing all 4 weekly questionnaires, and for the subsample of 198 patients who reported no opioid use preoperatively. In all models, greater predischarge opioid use predicted significantly increased likelihood of high opioid use postdischarge after adjusting for acetaminophen use before discharge: each 7.5 MMEs (equivalent to 1 oxycodone 5-mg tablet) taken in the 24 hours preceding discharge increased the likelihood of using more than 75 MMEs postdischarge by 9% (adjusted risk ratio = 1.09). Patients who reported low opioid use after discharge took on average 44% fewer MMEs in the 24 hours before discharge when compared with those who reported high opioid use after discharge (33.0 vs 59.3 MMEs, P <.001) (Table 1). In this adjusted analysis, acetaminophen use in the 24 hours before discharge was no longer significantly associated with an increased likelihood of high opioid use postdischarge.
Predictors of High Opioid Use Postdischarge
DISCUSSION
Key Findings
In this prospective cohort study of patients undergoing a cesarean delivery, we demonstrated a significant association between opioid use in the 24 hours before discharge and reported opioid use in the 4 weeks after discharge: patients who took fewer opioids predischarge also reported less opioid intake postdischarge. Meanwhile, patients received a median prescription of 225 MMEs at discharge, with no correlation between the discharge opioid prescription amount and predischarge opioid use. Although low users of opioids postdischarge took 44% fewer opioids before discharge, they received the same prescription as high users. Other studies have also shown that discharge opioid prescriptions do not correlate with predischarge opioid use or predischarge pain scores.17,18 Here, however, we quantify the strength of the relationship between predischarge and postdischarge opioid use. Our results permit design of practical individualized prescribing practices that may help reduce the large pool of excess opioids within our communities. In our cohort alone, there were 1,805 leftover opioid pills, averaging 9 pills per patient.
Even more troubling, only 16% of patients reported storing their leftover opioids in a locked location. These concerning opioid storage practices have been observed across different procedures and surgical subspecialties.8,9 For patients undergoing cesarean delivery, unlocked opioids pose an even greater threat given the presence of an infant and potentially other young children in the home. Merely 4% of patients reported disposing of their leftover opioid pills, resulting in 1,462 pills stored unlocked in the homes of patients in this study. This situation represents a substantial potential risk to children, family members, and visitors in the home given the potential for accidental ingestion or diversion of unused opioids. It is critical to establish better prescribing guidelines and practices in order to minimize this potential harm in our communities.
Other recent studies have attempted to identify factors that may help predict postdischarge opioid use in patients having a cesarean delivery. In a retrospective cohort study of 141 postcesarean patients, Schmidt et al33 found that longer operative time and increased number of opioid pills prescribed both independently correlated with top-quartile opioid use after discharge.33 This finding further enhances the concern about prescribing too many opioids, if in fact patients who receive more also take more. In another prospective cohort study of 179 postcesarean patients, Osmundson et al16 identified tobacco use and predischarge MMEs used as independent predictors of top-quartile use after discharge. They found, however, that only top-quartile opioid users took more MMEs per hour of inpatient stay than “average” opioid users. The top quartiles of cumulative postdischarge opioid use in the aforementioned studies were 45 opioid tablets33 and 200 MMEs,16 respectively. These values compare with a top quartile of 202.5 MMEs for the entire sample in our study. The higher amount of cumulative opioids reported taken in the study by Schmidt et al33 may be related to the fact that this study was the oldest (data collected from 2015-2016) and opioid prescription amounts have been down-trending in recent years.
Evidence-based guidelines have been shown to reduce the degree of overprescribing in the postoperative setting.34 To date, however, there are no such guidelines specifically for patients having a cesarean delivery. One recent publication from an expert panel recommends prescribing 0 to 10 oxycodone 5-mg tablets (0 to 75 MMEs) at discharge after a cesarean birth.20 In our cohort, patients reportedly took on average 90 MMEs in week 1 alone, and up to 13% of patients reported not taking opioids because they did not have any left over. This group represents patients with potential undertreatment of their pain, particularly if standard recommendations are enforced regardless of patient needs. Undertreatment of pain in postcesarean patients carries marked consequences that can affect both the mother and infant.12–14 To appropriately tailor opioid prescribing, opioid use in the 24 hours before discharge can be operationalized to determine the discharge prescription amount. For example, on the basis of the results of our regression model, a patient known to have taken 45 MMEs in the 24 hours before discharge could be expected to have a 54% increased likelihood of using more than 75 MMEs of opioid intake after discharge. Also, as most patients experience a rapidly tapering opioid requirement, weekly rather than monthly prescription plans may be more appropriate.
In our cohort, patients who reported a high level of opioid use after discharge were more likely to have received acetaminophen in the 24 hours before discharge. We believe this association reflects a difference in the baseline pain of the 2 groups, as patients with more pain would be more likely to receive acetaminophen and take more opioids. Indeed, acetaminophen should be used as a component of balanced analgesia in all patients unless contraindicated. This approach is supported by multiple professional guidelines recommending the use of nonopioid analgesics in managing postoperative pain.21,22 In our joint model that adjusted for predischarge acetaminophen use, opioid intake in the 24 hours before discharge remained the only highly significant predictor of higher opioid use in the 4 weeks after discharge.
After discharge, 69% of patients reported taking NSAIDs (eg, ibuprofen), 23% reported taking additional over-the-counter acetaminophen, and only 16% reported taking both. Multiple studies have shown the opioid-sparing effects of these agents independently.35–37 Further studies have even shown additional analgesic effects when acetaminophen and NSAIDs are combined.38,39 In our cohort, patients received combination pills containing both an opioid and acetaminophen 81% of the time, as opposed to opioid-only pills. The majority of patients reported not taking additional over-the-counter acetaminophen. In 2014, the US Food and Drug Administration limited the amount of acetaminophen to 325 mg in all opioid-acetaminophen combination pills and recommended a maximum daily acetaminophen dose of 3,000 mg.40 Patients would have to take more than 9 combination opioid pills per day to approach this acetaminophen limit. Most postcesarean patients take only 1 to 2 opioid pills per day and report not taking additional acetaminophen. Uncoupling opioids and acetaminophen may allow for maximizing of nonopioid analgesics first, thereby reducing the overall opioid requirements.
Limitations
Our cohort included all patients undergoing cesarean delivery with few exclusion criteria; however, this study is subject to several limitations. First, external validity may be limited by the fact that all patients were recruited at a single tertiary hospital. Regional variability in patient demographics and prescribing practices may limit the generalizability of our findings. Also, these results are applicable only to postcesarean patients and not to other surgical populations. Second, in addition to social desirability bias, there is a potential for recall bias given that postdischarge opioid use was collected from patient report. We attempted to minimize recall bias by sending 4 weekly questionnaires rather than 1 questionnaire at the end of 4 weeks. Third, nonresponse bias is a potential confounder for any survey study. Our response rate of 87% provides reasonable assurance, however, that such bias is not a major issue in this study.
Summary
In summary, we found that opioid use following discharge after a cesarean delivery is strongly associated with a patient’s intake of opioids and acetaminophen in the 24 hours before discharge. This information may help develop individualized opioid-prescribing strategies. Nonopioid analgesics that are effectively maximized for inpatients should also be maximized in the postdischarge period to improve pain management after cesarean delivery. Additional studies are needed to establish evidence-based guidelines for balanced oral analgesic regimens for postcesarean patients. Clinician education will be essential in changing opioid-prescribing practices, which currently vary widely between clinicians and institutions. Future studies will need to evaluate the impact of implementing evidence-based interventions on both clinician prescribing practices and potential undertreatment of pain.
Footnotes
Conflicts of interest: authors report none.
To read or post commentaries in response to this article, see it online at http://www.AnnFamMed.org/content/18/2/118.
Funding support: This work was supported by the National Institutes of Health (NIH), Award Number K23DA040923 to Karsten Bartels and NIH Award Number UL1TR002535.
Disclaimer: The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The NIH had no involvement in study design, collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
- Received for publication March 18, 2019.
- Revision received July 23, 2019.
- Accepted for publication August 14, 2019.
- © 2020 Annals of Family Medicine, Inc.