Abstract
PURPOSE Acne is an extremely common skin disease with an estimated global prevalence of 9.4%. We aim to provide comprehensive comparisons of the common pharmacological treatments for acne.
METHODS Randomized controlled trials comparing the efficacy of pharmacological therapies for acne vulgaris in patients of any age and sex and with a treatment duration of >2 weeks were included. PubMed and Embase databases were searched from inception until February 2022. Our prespecified primary end points were mean percentage reduction in total, inflammatory, and noninflammatory lesions. Treatment ranking was determined by P values.
RESULTS There were 210 articles describing 221 trials and 37 interventions included in the analysis. Our primary analysis of percentage reduction in total lesion count had 65,601 patients enrolled. Across all trials, the mean age was 20.4 years. The median duration of treatment was 12 weeks. The median total, inflammatory, and noninflammatory lesion counts were 72, 27, and 44, respectively. The most effective treatment was oral isotretinoin (mean difference [MD] = 48.41; P = 1.00), followed by triple therapy containing a topical antibiotic, a topical retinoid, and benzoyl peroxide (BPO) (MD = 38.15; P = .95) and by triple therapy containing an oral antibiotic, a topical retinoid, and BPO (MD = 34.83; P = .90). For monotherapies, oral or topical antibiotics or topical retinoids have comparable efficacy for inflammatory lesions, while oral or topical antibiotics have less effect on noninflammatory lesions.
CONCLUSION The most effective treatment for acne is oral isotretinoin, followed by triple therapies containing a topical retinoid, BPO, and an antibiotic. We present detailed comparisons of each intervention to serve as a practical database.
INTRODUCTION
Acne is an extremely common skin disease with an estimated global prevalence of 9.4% and an annual cost of 3 billion dollars in the United States.1,2 Measured by disability-adjusted life years, the global burden from acne is ranked the second highest among all cutaneous diseases worldwide.3 Acne commonly occurs on the face and upper trunk and can be categorized into inflammatory (ie, papules, pustules, nodules, and cysts) or noninflammatory lesions (ie, open or closed comedones).
A previous network meta-analysis for acne vulgaris published by Shi et al found that pharmacological interventions are generally more effective than non-pharmacological interventions.4 The spectrum of medications used to treat acne vulgaris includes topical or oral antibiotics, topical retinoids, oral isotretinoin (eg, Accutane [Roche Holding AG]), hormonal treatments (eg, combined oral contraceptives [COCs], topical clascoterone), benzoyl peroxide (BPO), azelaic acid (AA), and others.
Although medications recommended by various guidelines are generally supported by high-quality randomized controlled trials, many controversies and uncertainties still exist about comparisons between treatment options and there are markedly inconsistent drug prescribing patterns among countries and among prescriber specialties.5-8 A network meta-analysis conducted by Stuart et al focused on topical therapies for mild-to-moderate acne, but the number of interventions in their analysis was limited.9
In this network meta-analysis, we aim to provide a more comprehensive and detailed comparison of the common pharmacological treatments for acne, which include oral and topical medications as single or combined treatments.
METHODS
Selection Criteria
Only randomized controlled trials comparing the efficacy of pharmacological therapies for acne vulgaris were included. Split-face studies were also eligible. Patients of any age and sex with a diagnosis of acne vulgaris (from a clinical diagnosis or based on validated diagnostic criteria) and with a treatment duration longer than 2 weeks were included. Trials that included diseases other than acne vulgaris (eg, acne rosacea) must have reported results separately for acne vulgaris for inclusion in this study.
Studies must have reported at least 1 of the following end points: percentage or absolute decrease in either total, inflammatory, or noninflammatory lesions, or the proportion of participants achieving treatment success defined by the Investigator’s Global Assessment (IGA). Trials published only as abstracts without additional data sources were excluded. No language restrictions were applied.
The main interventions of interest were single or combination therapies of oral antibiotics, topical antibiotics, topical retinoids, oral isotretinoin, hormonal agents (ie, COCs, topical clascoterone), BPO, and AA. Uncommon medications with fewer than 3 trials or 200 participants were excluded.
Literature Search
We searched PubMed and Embase databases from inception to February 2022 with a combination of key words for article types and 6 fields (acne vulgaris, antibiotics, retinoid, hormonal therapy, benzoyl peroxide, and azelaic acid). Free-text terms were searched in complement with medical subject headings (MeSH) terms or Emtree (Embase’s unique subject headings) terms (Supplemental Table 1). Reference lists of all included articles were also screened.
Selection Process and Data Extraction
Two researchers (C-Y.H. and T-S.H.) independently assessed all trials according to the predefined selection criteria. Any disagreement was resolved through discussion with a third researcher (C-C.L.). We extracted trial design, trial size, details of intervention (including route, dose, frequency, and treatment duration), patient characteristics (eg, mean age, sex, and baseline lesion counts), and outcome data for each time point. For crossover trials, only data from the first period were extracted to avoid possible carryover effects. For split-face studies, the lesion counts were multiplied by 2. Outcome data were approximated from the figure when no precise numerical data were provided. Standard deviations were calculated or imputed from standard errors, 95% CIs, or P values when necessary, according to the Cochrane handbook.10
End Points
The primary end points were mean percentage reduction in total, inflammatory, and noninflammatory lesions. Mean absolute reduction in total, inflammatory, and noninflammatory lesions were also analyzed as secondary outcomes. Direct measurements of mean percentage reduction and mean absolute reduction were preferred, followed by approximations based on baseline lesion counts. Another efficacy end point was the odds ratio of patients achieving treatment success, defined by an improvement of 2 grades from baseline and/or reaching clear or almost clear on the IGA of acne severity. An FDA guideline recommended the definition of success for the IGA scale.11 The IGA is an ordinal scale with 5 severity grades (clear, almost clear, mild, moderate, and severe) that assesses overall acne severity. The safety end point was measured by the odds ratio of patients discontinuing treatment due to adverse events.
Risk of Bias Assessment
The methodological quality of included trials were assessed by the 2 investigators (C-Y.H. and T-S.H.) using a slightly adapted version of the risk of bias approach of the Cochrane Collaboration (Supplemental Table 2).10 A number of key components were evaluated, including the randomization process, deviations from the intended interventions (blinding of the participants), measurement of the outcome (blinding of the investigator), and missing outcome data. We skipped the question regarding the selection of the reported results, since the outcomes and analyses of the acne clinical trials were relatively straightforward and we did not identify any trials with a high risk of bias in this regard.
Statistical Analysis
We performed the network meta-analysis using frequentist methods,12 and adopted a random-effects model. There were 37 treatment nodes included in the primary analysis, which included the 1 reference treatment (ie, placebo). For the percentage and absolute reductions in total, inflammatory, and noninflammatory lesion counts, mean differences (MD) between the effect sizes were computed using a restricted maximum likelihood estimation for each comparison of 2 treatments. For the success rate, defined by IGA, and the safety end point (ie, discontinuation due to adverse events), odds ratios were computed with 95% CI. P values were calculated based on the point estimates and standard errors of the network estimates.
Global heterogeneity was assessed by I-square statistics, which described the percentage of variation across studies due to heterogeneity rather than chance. We also assessed the inconsistency between direct and indirect comparisons by a node-splitting method.13 The hot spots of inconsistency were located and visualized by heat plots.14 We checked for potential publication bias and small-study effects by the comparison-adjusted funnel plots with the specified order by the earliest publication year of each treatment.15 We also conducted a separate analysis to evaluate the efficacy of more general treatment types by pooling the treatment nodes with similar mechanisms (eg, oral antibiotics, topical antibiotics, topical retinoids). Two additional sensitivity analyses were performed by excluding studies before 1985 and by excluding studies with low quality scores (score 1 or 2). The year 1985 was chosen because, until that year, the lesions of acne vulgaris were not well stratified into inflammatory vs noninflammatory lesions in clinical trials. All computations were conducted in the software R, version 3.1.1 (R Foundation for Statistical Computing) with the package netmeta.16
RESULTS
Search Results
Our search yielded 1,280 articles from PubMed and 2,061 articles from Embase. After removing 689 duplicates, 2,652 articles were screened for titles and abstracts. With 7 additional articles identified from the reference lists, 739 articles entered full-text review. Finally, 210 articles describing 221 trials were eligible for inclusion (Figure 1).
Flow diagram of selection of included articles.
Study Characteristics and Quality Assessment
In total, there were 65,601 patients in our primary analysis of percentage reduction in total lesion count. Across all trials, the mean age was 20 years (range, 10-38 years). The median duration of treatment was 12 weeks (range, 2-48 weeks). The median total, inflammatory, and noninflammatory baseline lesion counts were 71.5, 27, and 44, respectively (Supplemental Table 3). The 37 treatment nodes in our network meta-analysis, included 6 oral antibiotics, 5 topical antibiotics, oral isotretinoin, 5 topical retinoids, 6 COCs, topical clascoterone, 10 combination therapies, BPO, AA, and placebo (Supplemental Figure 1a, Supplemental Table 4). We categorized tretinoin and isotretinoin as first-generation topical retinoids and tazarotene and adapalene as second generation based on their molecular structure and receptor selectivity.17
Of all trials, 194 (88%) were investigator blinded and 130 (58%) were double blinded (Supplemental Table 5). Seventy-four (34%) trials reported appropriate random sequence generation and 136 (62%) trials reported reasons for withdrawals with similar proportions of missing data between groups.
Comparative Efficacy
Total Lesion Count
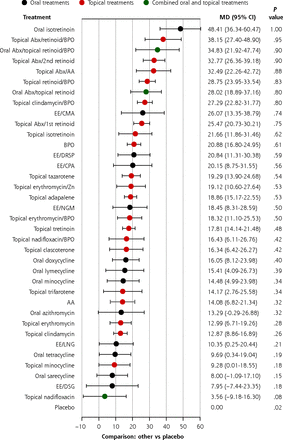
For the network of percentage reduction in total lesion count (190 studies) the mean differences in percentage reduction compared with placebo were ranked by forest plot (Figure 2a). The most effective treatment is oral isotretinoin (MD = 48.41, P = 1.00). Apart from oral isotretinoin, combination therapies appeared to be more effective than monotherapies. Triple therapy containing a topical antibiotic, a topical retinoid, and BPO was the second most effective treatment (MD = 38.15, P = .95), followed by triple therapy comprised of an oral antibiotic, a topical retinoid, and BPO (MD = 34.83, P = .90). The combinations of a topical antibiotic with a topical retinoid or AA are also highly effective. Among topical retinoids, isotretinoin was the most effective (MD = 21.66, P = .62), followed by tazarotene (MD = 19.29, P = .54).
Estimates of the percentage reduction in total lesion count for different treatments compared with placebo in the primary analysis.
AA = azelaic acid; Abx = antibiotic; BPO = benzoyl peroxide; CMA = chlormadinone acetate; CPA = cyproterone acetate; DRSP = drospirenone; DSG = desogestrel; EE = ethinyl estradiol; LNG = levonorgestrel; MD = mean difference; NGM = norgestimate; Zn = zinc.
Note: Forest plots of MD and 95% CI in percentage reduction of compared with placebo in a random effects model.
Antibiotic monotherapies were generally less effective than other therapies. Among antibiotics, oral doxycycline (MD = 16.05, P = .40) and oral lymecycline (MD =15.41, P = .39) ranked among the top, while topical nadifloxacin (MD = 3.56, P = .08), oral sarecycline (MD = 8.00, P = .15), topical minocycline (MD = 9.28, P = .18), and oral azithromycin (MD = 13.29, P = .32) did not demonstrate significant superiority when compared with placebo. Of note, while topical clindamycin alone was less effective than other antibiotics (MD = 12.87, P = .26), the addition of BPO significantly boosted its efficacy (MD = 27.29, P = .80).
The efficacy of COCs ranged widely, with ethinyl estradiol/chlormadinone acetate being the most effective (MD = 26.07, P = .74). Topical clascoterone, a first-in-class androgen receptor inhibitor, had a modest effect (MD = 16.34, P = .42).
All interventions compared with each other are shown in a league table (Supplemental Table 6). A similar ranking of treatment efficacy was observed in the network of absolute reduction in total lesion count, which included 176 studies (Supplemental Figure 2a and Supplemental Figure 3a).
Inflammatory Lesion Count
The network of percentage reduction in inflammatory lesion counts included 204 studies (Supplemental Figure 1b). Except for ethinylestradiol/desogestrel, all other 35 treatments were significantly better than placebo (Figure 2b). The most effective intervention was oral isotretinoin (MD = 54.22, P = 1.00), followed by topical antibiotics plus AA (MD = 43.62, P = .96), oral antibiotics with topical retinoid and BPO (MD = 36.96, P = .91), and topical antibiotics with topical retinoid and BPO (MD = 33.04, P = .86). Various dual therapies with any 2 of antibiotics (oral or topical), topical retinoid, and BPO were also among the top of the list. Among oral antibiotics, doxycycline (MD = 22.98, P = .57) ranked the highest. Monotherapies of topical retinoid or antibiotics alone were similarly less effective.
Estimates of the percentage reduction in inflammatory lesion count for different treatments compared with placebo in the primary analysis.
AA = azelaic acid; Abx = antibiotic; BPO = benzoyl peroxide; CMA = chlormadinone acetate; CPA = cyproterone acetate; DRSP = drospirenone; DSG = desogestrel; EE = ethinyl estradiol; LNG = levonorgestrel; MD = mean difference; NGM = norgestimate; Zn = zinc.
Note: Forest plots of MD and 95% CI in percentage reduction compared with placebo in a random effects model.
The efficacy of COCs was highly variable, with ethinyl estradiol/chlormadinone acetate being the most effective (MD = 26.22, P = .67). Head-to-head comparisons of each intervention were made (Supplemental Table 7). The network of absolute reduction in inflammatory lesion counts (186 trials) generally recapitulated the ranking (Supplemental Figure 2b and Supplemental Figure 3b).
Noninflammatory Lesion Count
A total of 187 studies were included in the network of percentage reduction in noninflammatory lesions (Supplemental Figure 1c). The top 3 interventions were oral isotretinoin (MD = 48.47, P = 1.00), topical antibiotics with topical retinoid and BPO (MD = 32.65, P = .95), and oral antibiotics with topical retinoid and BPO (MD = 30.02, P = .90) (Figure 2c).
Estimates of the percentage reduction in noninflammatory lesion count for different treatments compared with placebo in the primary analysis.
AA = azelaic acid; Abx = antibiotic; BPO = benzoyl peroxide; CMA = chlormadinone acetate; CPA = cyproterone acetate; DRSP = drospirenone; DSG = desogestrel; EE = ethinyl estradiol; LNG = levonorgestrel; MD = mean difference; NGM = norgestimate; Zn = zinc.
Note: Forest plots of MD and 95% CI in percentage reduction compared with placebo in a random effects model.
Regarding monotherapies, all topical retinoids ranked better than any of the oral or topical antibiotics. The most effective topical retinoid was tazarotene (MD = 22.61, P = .76) followed by tretinoin (MD = 19.22, P = .64). Topical nadifloxacin, oral minocycline, oral sarecycline, and oral lymecycline, were not statistically superior compared with placebo.
As for the inflammatory lesions, ethinyl estradiol/chlormadinone acetate was the most effective among COCs (MD = 25.76, P = .82). Comparisons of all interventions with each other are presented in league table (Supplemental Table 8). The network of absolute reduction in non-inflammatory lesion counts consisted of 169 studies and yielded similar results (Supplemental Figure 2c and Supplemental Figure 3c).
Network With Simplified Intervention Nodes
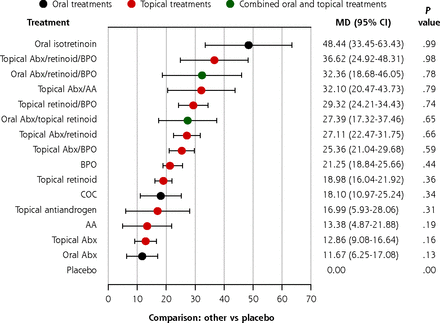
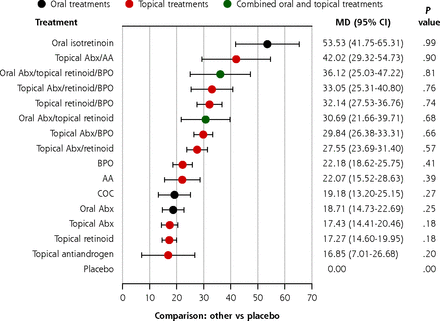
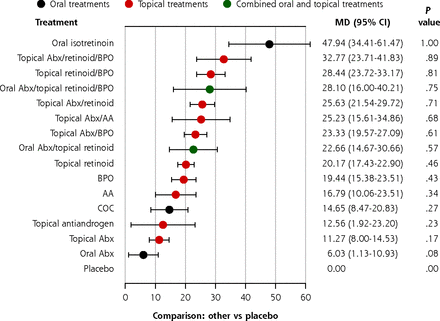
We reduced the number of intervention nodes by pooling treatments with similar mechanisms together. In this sensitivity analysis, 16 intervention nodes were included (Supplemental Figure 4, Supplemental Table 4). The overall trends in the simplified network (Figure 3) were similar to the primary analysis for total, inflammatory, and noninflammatory lesions. Oral isotretinoin was the most effective treatment, followed by various combination therapies. Other monotherapies were the least effective. Importantly, a topical retinoid monotherapy was still significantly better than a topical or oral antibiotic monotherapy in reducing noninflammatory lesion counts. Pairwise comparisons of all intervention nodes were made (Supplemental Tables 9, 10, and 11).
Forest plot estimates of the percentage reduction in total lesion count compared with placebo in the simplified network.
AA = azelaic acid; Abx = antibiotic; BPO = benzoyl peroxide; COC = combination oral contraceptives; MD = mean difference.
Note: Forest plots of MD and 95% CI in percentage reduction compared with placebo in a random effects model.
Estimates of the percentage reduction in inflammatory lesion count compared with placebo in the simplified network.
AA = azelaic acid; Abx = antibiotic; BPO = benzoyl peroxide; COC = combination oral contraceptives; MD = mean difference.
Note: Forest plots of MD and 95% CI in percentage reduction compared with placebo in a random effects model.
Estimates of the percentage reduction in noninflammatory lesion count compared with placebo in the simplified network.
AA = azelaic acid; Abx = antibiotic; BPO = benzoyl peroxide; COC = combination oral contraceptives; MD = mean difference.
Note: Forest plots of MD and 95% CI in percentage reduction compared with placebo in a random effects model.
Treatment Success Measured by Investigator’s Global Assessment
The network of treatment success evaluated by IGA consisted of 69 trials (Supplemental Figure 5a). Similar to the trend for the primary end point, triple therapies containing a oral/topical antibiotic, a topical retinoid, and BPO remained among the top-ranked treatments with the highest P values, and combination therapies were generally more effective than the monotherapies (Figure 4a). Unlike the primary end point trend, oral isotretinoin did not appear among the top treatments in the IGA analysis. A possible explanation was that there were substantially fewer trials in the IGA analysis, especially for oral isotretinoin, which led to a wider 95% CI.
Estimates of the treatment success evaluated by Investigator’s Global Assessment compared with placebo.
AA = azelaic acid; Abx = antibiotic; BPO = benzoyl peroxide; DRSP = drospirenone; EE = ethinyl estradiol; IGA = Investigator’s Global Assessment; LNG = levonorgestrel; OR = odds ratio.
Note: Forest plots of OR and 95% CI for treatment success measured by IGA compared with placebo in a random effects model.
Discontinuation Due to Adverse Events
A total of 132 trials were analyzed in the network of discontinuation due to adverse events (Supplemental Figure 5b). The odds ratios of discontinuation did not differ significantly from placebo for most (26 out of 35) interventions (Figure 4b). Topical trifarotene had the highest odds ratio for discontinuation, followed by topical tazarotene and the combination of a topical retinoid and BPO. The discontinuation rates remained low, however, even for topical trifarotene (2%).18
Estimates of the discontinuations due to adverse events compared with placebo.
AA = azelaic acid; Abx = antibiotic; AE = adverse event; BPO = benzoyl peroxide; CMA = chlormadinone acetate; CPA = cyproterone acetate; DRSP = drospirenone; DSG = desogestrel; EE = ethinyl estradiol; LNG = levonorgestrel; NGM = norgestimate; OR = odds ratio; Zn = zinc.
Note: Forest plots of OR and 95% CI for discontinuations due to AEs compared with placebo in a random effects model.
Heterogeneity, Inconsistency, and Publication Bias
We found a moderate-to-high global heterogeneity of the studies among the networks. For the networks of percentage reduction of lesion counts, the I2 statistic was 79% for total lesions, 67% for inflammatory lesions, and 61% for noninflammatory lesions. In the node-splitting analysis, only a few pairs of comparison showed a significant inconsistency between direct and indirect evidence (9 of 111, 9 of 118, and 9 of 110 pairwise comparisons in the networks of percentage reduction in total, inflammatory, and noninflammatory lesion counts, respectively) (Supplemental Figures 6, 7, and 8). The net heat plot assessing the contribution of each study design revealed few localized hot spots of inconsistency (Supplemental Figures 9, 10, and 11). No strong publication bias was found via the visual inspection in the funnel plots with no obvious asymmetry of all networks (Supplemental Figure 12).
Sensitivity Analysis
The first sensitivity analysis excluded 12 studies published before 1985 (Supplemental Figure 13). The overall treatment ranking for percentage reduction in total, inflammatory, and noninflammatory lesion counts remained largely unchanged (Supplemental Figure 14).
The second sensitivity analysis excluded 35 studies with low quality scores (1 or 2 out of 5) (Supplemental Figure 15). The efficacy of most treatments was also similar to that of the primary analysis (Supplemental Figure 16). The only difference was that the combination of nadifloxacin and BPO appeared among the best options for reducing total lesion count (MD = 39.12, P = .91; ranked 3rd) and inflammatory lesion count (MD = 50.33, P = .96; ranked 2nd). Only 2 studies, however, were left for this intervention, and the 95% CIs were wide. As topical nadifloxacin is primarily approved in Japan and Europe, but not in the United States, combination therapies that contain it require more high-quality studies to confirm their efficacy.
DISCUSSION
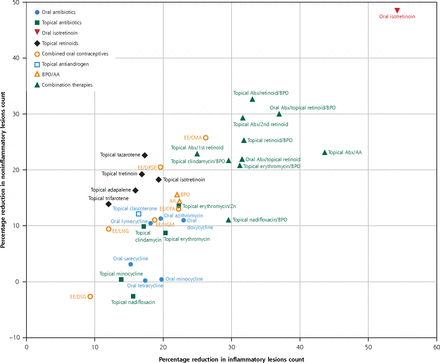
Our network meta-analysis is the largest study to date providing broad and detailed comparative efficacy of pharmacological interventions in acne vulgaris for the reduction of inflammatory or noninflammatory acne lesions (Figure 5). Oral isotretinoin is the most effective treatment, followed by combination therapies consisting of an oral or topical antibiotic, topical retinoid, and BPO. In general, oral antibiotics, topical antibiotics, and topical retinoids as monotherapies have comparable efficacy on inflammatory lesion counts. For noninflammatory lesions, topical retinoids are significantly more effective, and oral antibiotics alone are inadequate treatments. The efficacies of different COCs are generally modest. The combination of a topical retinoid and BPO is at least equally effective as an oral antibiotic with a topical retinoid in reducing inflammatory lesion counts. This information may change treatment strategies by reducing the need of oral antibiotic treatment in inflammatory acne, thus minimizing the risk of antibiotic resistance.
Biplots of reduction in inflammatory and noninflammatory lesion counts in the primary analysis.
AA = azelaic acid; Abx = antibiotic; BPO = benzoyl peroxide; CMA = chlormadinone acetate; CPA = cyproterone acetate; DRSP = drospirenone; DSG = desogestrel; EE = ethinyl estradiol; LNG = levonorgestrel; NGM = norgestimate; Zn = zinc.
Note: Percentage reduction in inflammatory lesion count (x axis) vs percentage reduction in noninflammatory lesion count (y axis) compared with placebo.
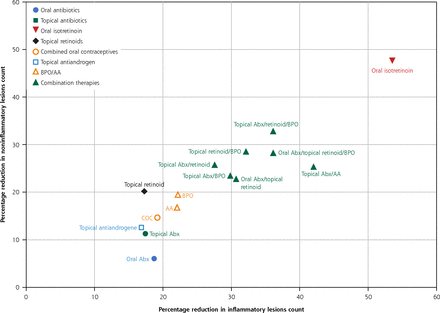
Bioplots of reduction in inflammatory and noninflammatory lesion counts in the simplified model.
AA = azelaic acid; Abx = antibiotic; BPO = benzoyl peroxide; COC = combined oral contraceptives.
Note: Percentage reduction in inflammatory lesion count (x axis) vs percentage reduction in noninflammatory lesion count (y axis) compared with placebo.
The network meta-analysis published by Shi et al concluded that combining a topical retinoid with BPO was the most effective treatment.4 They provided effect estimates only for treatment categories (eg, topical retinoid), however, not for individual treatment (eg, tazarotene, adapalene). Moreover, only absolute inflammatory and noninflammatory lesion counts were analyzed in their work. Another network meta-analysis by Stuart et al focused on topical therapies found that adapalene with BPO was the most effective.9 However, they left out several commonly prescribed medications. For example, they included only adapalene but not tazarotene. Newer treatments, such as trifarotene and clascoterone, were also not included in their work. Our study includes 3 to 4 times as many trials as these previous works. We agree with some of their findings, ie, that topical clindamycin with BPO combination therapy or BPO monotherapy are more effective than topical clindamycin monotherapy, especially for noninflammatory lesions. Our results, however, also indicate that besides oral isotretinoin, triple-therapy with an oral or topical antibiotic, a topical retinoid, and BPO is even more effective in reducing total lesion counts compared with the dual therapy of a topical retinoid with BPO. We also found that other dual therapies, such as topical clindamycin with topical adapalene or topical clindamycin with AA (not included in prior meta-analyses) are equally as effective as the topical adapalene with BPO combination. Furthermore, we emphasize that oral and topical antibiotics alone are of limited effectiveness and should be avoided. Our results are more robust than the previous network meta-analysis because multiple outcomes (percentage or absolute reduction in total, inflammatory, noninflammatory lesion counts; IGA; and discontinuation due to adverse events) are examined and several sensitivity analyses (network with simplified treatment nodes, exclusion of the older studies, exclusion of the studies with low quality scores) are performed.
Although the rates of drop out due to adverse events are generally low (<2%), the severity of adverse events differs and must be taken into consideration in clinical practice. Adverse reactions to topical medications, such as erythema, dryness, peeling, itching, and stinging, are mostly mild to moderate.19 Combining multiple topical medications may increase skin irritation.20,21 Local side effects or discontinuation due to adverse events were more commonly observed for the topical adapalene with BPO combination group than other combinations (eg, topical clindamycin with BPO).22,23 Common side effects of oral isotretinoin are mucocutaneous (eg, dry lip, dry skin, cheilitis), whereas oral tetracyclines may cause nausea, vomiting, and abdominal pain.24-27 Although not reflected in the statistical analysis on discontinuation rates, some studies reported the potential serious adverse events of oral isotretinoin. For example, in Tan’s study, 5 treatment-related severe adverse events (ie, dry lips, fatigue, acne flare), including one related serious adverse event (Stevens–Johnson syndrome), were reported in the oral isotretinoin group.28 The major concerns of COCs are venous thromboembolic events and myocardial infarction.
Our study limitations include the inability to separately analyze specific dosing schedules or formulations due to the paucity of the studies. For example, a modified-release dosage of doxycycline 40 mg may have comparable efficacy with traditional doxycycline 100 mg given once daily, or a solubilized gel formation of BPO may also enhance bioavailability, follicular penetration, and efficacy.24,29 The dose of oral isotretinoin is another critical issue. A systematic review recommended low-dose isotretinoin (0.1 to 0.3 mg/kg) due to fewer side effects (eg, cheilitis) and improved cost-effectiveness compared with conventional dose (0.5 to 1.0 mg/kg).30 In another meta-analysis, the authors concluded that low-dose isotretinoin was associated with a lower response rate and a higher relapse rate.31 Still, only a few trials (4 trials comparing response rate and 2 trials comparing relapse rate) were included, and the odds ratio was not statistically significant.31 More trials to compare different dosing regimens of oral isotretinoin are required. Furthermore, acne is a chronic problem requiring long-term follow-up, but the median study treatment duration was only 12 weeks with prior studies showing the deterioration of acne control occurs once the active treatment is discontinued.32 Lastly, a few discrepancies are noted among different analyses, such as oral isotretinoin in the IGA analysis and topical nadifloxacin in the sensitivity analysis excluding trials with low quality scores. Future high-quality trials of these treatments may help confirm their efficacy.
In conclusion, our study provides the most comprehensive evidence to date about common pharmacological interventions for acne vulgaris with analyses of both percentage and absolute reduction in lesion counts and detailed comparisons for each intervention. We confirmed that oral isotretinoin is the most effective acne treatment, followed by combination therapy consisting of an oral or topical antibiotic with topical retinoid and BPO. For monotherapies, oral and topical antibiotics and topical retinoids have comparable efficacy for inflammatory lesions, while oral and topical antibiotics are less effective for noninflammatory lesions and should not be used as monotherapy due to the risk of bacterial resistance developing.33 We present detailed comparisons of each intervention to serve as a practical database and to complement to current guidelines.
Acknowledgment
We thank Dr Julia Gao for editorial assistance.
Footnotes
Conflicts of interest: authors report none.
- Received for publication July 26, 2022.
- Revision received January 25, 2023.
- Accepted for publication January 31, 2023.
- © 2023 Annals of Family Medicine, Inc.