Abstract
PURPOSE To determine the effects of stratified primary care for low back pain (SPLIT program) in decreasing back-related disability for patients with low back pain (LBP) in primary care.
METHODS We conducted a before-and-after study. We compared health-related outcomes for 2 sequential, independent cohorts of patients with LBP recruited at 7 primary care units in Portugal. The first prospective cohort study characterized usual care (UC) and collected data from February to September 2018. The second was performed when the SPLIT program was implemented and collected data from November 2018 to October 2021. Between cohorts, physical therapists were trained in the implementation of the SPLIT program, which used the STarT Back Screening Tool to categorize patients for matched treatment. We compared back-related disability (Roland-Morris Disability Questionnaire, 0-24 points), pain (Numeric Pain Rating Scale, 0-10 points), perceived effect of treatment (Global Perceived Effect Scale, −5 to +5 points), and health-related quality of life (EuroQoL 5 dimensions 3 levels index, 0-1 points).
RESULTS We enrolled a total of 447 patients: 115 in the UC cohort (mostly treated with pharmacologic treatment) and 332 in the SPLIT cohort (all referred for a physical therapy intervention program). Over the study period of 6 months, patients in the SPLIT program showed significantly greater improvements in back-related disability (ß, −2.94; 95% CI, −3.63 to −2.24; P ≤ .001), pain (ß, −0.88; 95% CI, −1.18 to −0.57; P ≤ .001), perceived effect of treatment (ß, 1.40; 95% CI, 0.97 to 1.82; P ≤ .001), and health-related quality of life (ß, 0.11; 95% CI, 0.08 to 0.14; P ≤ .001) compared with UC.
CONCLUSIONS Patients in the SPLIT program for LBP showed greater benefits regarding health-related outcomes than those receiving UC.
- low back pain
- stratified care
- physical therapy
- primary health care
- family practice
- controlled before-after study
INTRODUCTION
Low back pain (LBP) is a high-burden health problem worldwide, and its management represents a serious challenge for health systems.1-4 The burden caused by LBP is mainly explained by the great disability it causes in a minority of patients, those with persistent, disabling pain.5,6
To decrease the number of patients who develop persistent, disabling LBP, the implementation of a stratified approach in primary care has been suggested.7-10 Using the STarT Back Screening Tool (SBST) to categorize patients according to low, medium, and high risk of developing poor disability, this approach suggests matched physical therapy treatments of increasing complexity according to risk subgroup.11
The STarT Back trial was the first study to show the effectiveness and cost-effectiveness of the stratified approach for LBP.12,13 Aiming to generalize these results, the IMplementation to improve Patient Care through Targeted treatment (IMPaCT) study confirmed the potential of this approach for improving patient-level outcomes while decreasing the consumption of health care in the routine clinical practice of a UK primary care setting.14,15
Considering that health systems are context specific and might vary substantially between countries, several studies sought to adapt and test the stratified approach in primary care.16-20 A country that might benefit from such a comprehensive approach is Portugal. In addition to being the major cause of disability-adjusted life-years,1 LBP management in Portuguese primary care is described as fragmented and not aligned with clinical recommendations.21,22 Given this scenario, we aimed to determine the effects of stratified primary care for low back pain (SPLIT) in decreasing back-related disability for patients seeking primary care for an LBP episode.
METHODS
Design and Setting
The SPLIT study used a before-and-after design embedded in Portuguese primary care.23 This is an integral part of the public National Health System and is geographically organized within regional health administrations that manage a set of health center groups, which in turn includes a set of health units to which residents are assigned. The present study was developed under a protocol with the Regional Health Administration of Lisbon and Tagus Valley (ARS-LVT) via its Arrábida health center group. With a total of 23 health units, this health center group is responsible for 242,928 patients from 3 geographic regions (Palmela, Sesimbra, Setubal).
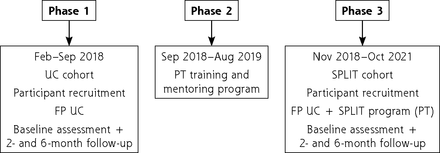
Two sequential, independent cohorts of patients with LBP were recruited at 7 health units according to the different study phases. These units were chosen considering the proportion of geographic area covered (Palmela, Sesimbra, Setubal) and the presence of primary care–based physical therapists (PTs) in these workplaces. In Phase 1, a cohort of patients (usual care [UC] cohort) was recruited to characterize UC and patients’ health-related outcomes (February-September 2018). Slightly before the end of this phase, a training and mentoring program (Phase 2) aiming to optimize the implementation of the SPLIT program in the routine clinical practice of the 4 participating PTs was developed and executed (September 2018-August 2019). In Phase 3, a new cohort of patients (SPLIT cohort) was recruited to evaluate the outcomes of the SPLIT program (November 2018-October 2021). The longer recruitment period of Phase 3 represents a deviation from the protocol and is mainly explained by the constraints associated with the coronavirus disease 2019 (COVID-19) pandemic. The decision to increase the recruitment period aimed to prevent access restrictions to health care during the pandemic from affecting the future maintenance of the SPLIT program. The study was approved by the Ethics Committee of ARS-LVT (REF 3562/CES/2018); its framework is presented in Figure 1.
Study framework.
FP = family physician; PT = physical therapist; SPLIT = stratified primary care for low back pain; UC = usual care.
Participants
Participants were eligible if they had consulted a participating family physician (FP) for nonspecific LBP24,25 of any duration, with or without leg pain; were aged 18-65 years; and were able to read and speak the Portuguese language. Exclusion criteria were clinical signs of infection, tumor, osteoporosis, fracture, inflammatory disorder, radicular syndrome, severe depression or other psychiatric condition, pregnancy, or having undergone back surgery or conservative treatment within the previous 6 or 3 months, respectively.
Phase 1: Usual Care Cohort
Patients from participating health units were recruited consecutively by their attending FP. Patients were invited to participate and asked to be contacted by a trained research assistant (RA), who confirmed eligibility criteria and informed them about the study. For those who consented, sociodemographic and clinical data (including back-related disability, pain, and health-related quality of life [HRQoL]) were collected by the RA by interview, using a standard data collection form.
In this phase, FPs were not aware of the SPLIT program; they were encouraged to manage patients as usual based on their clinical judgment. Monthly debriefing meetings were scheduled to motivate participating FPs regarding the recruitment process. The course of outcomes for this cohort is published elsewhere.21
Phase 2: Training and Mentoring Program
The SPLIT program consisted of a structured physical therapy program informed by the theoretical rationale and interventions suggested by the STarT Back Trial26 and other frameworks.27-29 After each patient’s initial FP consultation and referral, the following 3 main steps were taken: (1) clinical assessment by a trained PT, including use of the SBST,30 for stratification of risk of poor disability, (2) matched treatment, and (3) a monitoring program to track outcomes (Supplemental Appendix).
Considering the lack of involvement of primary care–based PTs within the clinical pathway for LBP and the potential challenges implementation of the SPLIT program would entail,31 we developed a training program for PTs, which comprised a 30-hour course delivered by 4 PTs with expertise in training clinicians. To consolidate the knowledge and confidence of participating PTs,31 we executed a mentoring program involving monthly contacts over a period of 12 months to provide peer feedback and clinical case review. To present the SPLIT program and motivate FPs regarding the recruitment and referral procedures, we organized 2 interactive group-based sessions, in which the best evidence on the effects of nonpharmacologic interventions for LBP was presented.7,8,32-34 Two rheumatologists and 2 PTs moderated these sessions. Monthly debriefing meetings continued to be scheduled.
Phase 3. SPLIT Cohort
Participating FPs from the same 7 health units were asked to keep the recruitment process used for Phase 1. They were again encouraged to manage patients as usual, except for the referral of potentially eligible participants who should be referred to primary care–based PTs for evaluation and treatment. After referral, participating PTs confirmed the eligibility criteria and informed patients about the study, inviting them to participate. For those who consented, sociodemographic and clinical data were collected using the same procedures as for Phase 1. The decision to entrust participating PTs with the responsibility of collecting patients’ data aimed to optimize the implementation of the SPLIT program. Because it included, for clinical reasoning purposes, the collection of clinical data that were used later as study outcomes (ie, back-related disability and pain intensity), this decision prevented the duplication of data collection and thus decreased patients’ burden with regard to answering questionnaires.
Health-Related Outcomes
Health-related outcomes were assessed at 2- and 6-month follow-ups (by telephone) by the RA (UC cohort) and participating PTs (SPLIT cohort). The use of telephone interviews was established to potentiate patient retention. Training was provided to standardize data collection procedures. The primary outcome was back-related disability measured with the Portuguese version of the Roland-Morris Disability Questionnaire (RMDQ) (0-24 points [greater scores indicating greater disability]).35-37 Secondary outcomes included pain intensity, HRQoL, and perceived effect of treatment. For pain intensity, the Portuguese version of the Numeric Pain Rating Scale (NPRS) was used (0 = no pain, 10 = worst possible pain).38-40 The HRQoL was measured with the EuroQol 5-dimension 3-level (EQ-5D-3L), a generic instrument suggesting an index of the individual health status (1 = the best possible health, 0 = death).41,42 The weight applied was based on the Portuguese valuation study.43 The Global Perceived Effect Scale (GPES) measured the perceived effect of treatment (−5 = vastly worse, +5 = completely recovered).44-46
Statistical Analysis
We used descriptive statistics to characterize both cohorts of participants. Baseline differences between cohorts were tested with the Mann-Whitney test (continuous variables) and the χ2 test (categorical variables).
We used linear mixed-effects models to compare cohorts regarding the RMDQ, NPRS, GPES, and EQ-5D-3L index over follow-up (ie, baseline, 2- and 6-month time points). Because we also considered the proportion of patients who met the minimum important change (MIC) criteria (ie, ≥30% decrease from baseline for RMDQ and NPRS and a GPES score ≥3)46,47 and those with poor disability (RMDQ ≥7),11 we also used logistic mixed-effects models. All of the models included varying intercepts for each participant and an identity covariance structure. For outcomes that were not measured at baseline (ie, GPES and MIC outcomes), the 2-month follow-up was considered the models’ first time point.
Given the observational nature of the study and the consequent probability of confounding, we adjusted models for baseline imbalances between cohorts (ie, age; referred leg pain; and baseline SBST psychosocial subscale, NPRS, RMDQ, and EQ-5D-3L values) and clinically important characteristics (ie, duration of LBP episode). Despite some of these measures having been collected at all 3 time points, they were entered into the models as time independent to address baseline discrepancies.
We conducted a sensitivity analysis to investigate whether the deviation from the protocol regarding recruitment time influenced the results. This followed the procedures described for the main analysis; however, for the SPLIT cohort, only those participants recruited from September 2018 to August 2019 were considered. For exploratory purposes, we also conducted a descriptive analysis of outcomes considering the SBST risk subgroups (low, medium, and high risk). STarT Back Screening Tool scoring methods are presented in the Supplemental Appendix. Given the low proportion of missing data, statistical methods for handling missing date were not considered. A significance level of 5% was used. All analyses were performed with SPSS Statistics version 23 (IBM).
RESULTS
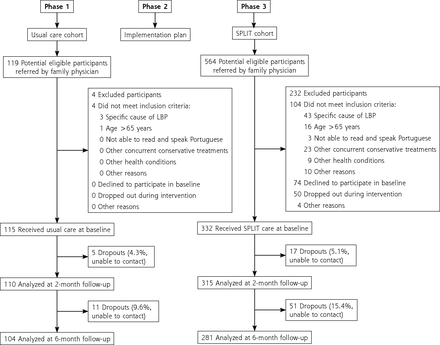
A total of 447 participants were included in the study: 115 in the UC cohort and 332 in the SPLIT cohort (Figure 2). For the SPLIT cohort, most of the included participants (173 [52.1%]) were referred during the first 12 months. The remaining 78 (23.5%) and 81 (24.4%) were referred during the second and third years of recruitment, respectively.
Study flow diagram.
LBP = low back pain; SPLIT = stratified primary care for low back pain.
In Phase 1 (UC cohort), patients were predominantly treated by FPs with pharmacologic treatment (85.3%). Nonsteroidal anti-inflammatory drugs (81.4%) and muscle relaxants (60.8%) were the most common, followed by weak opioids (19.6%). Only 8.3% of patients were referred for physical therapy, namely to community clinics not related to primary care. In Phase 3, all patients in the SPLIT cohort were referred to trained primary care–based PTs. A mean (SD) of 3.76 (3.70) physical therapy sessions were provided: 1.11 (0.71) for patients with low risk, 4.48 (2.52) for medium risk, and 9.98 (3.31) for high risk.
Participant Characteristics
Participants had a mean (SD) age of 46.19 (11.65) years and were predominantly female (268 [60.0%]) and employed (314 [70.7%]) (Table 1). Except for the older age of the SPLIT cohort (P = .035), sociodemographic characteristics were similar between cohorts. Clinically, LBP-related symptoms were generally less severe in participants in the SPLIT cohort (P ≤ .001); they had less referred leg pain, risk of poor disability, back-related disability, pain, and increased HRQoL compared with the UC cohort.
Baseline Characteristics of Participants
Health-Related Outcomes
With respect to the 6-month follow-up period, there was a significant and superior improvement in back-related disability for the SPLIT cohort compared with the UC cohort (ß, −2.94; 95% CI, −3.63 to −2.24; P ≤ .001) (Table 2 and Table 3). The superiority of changes favoring the SPLIT program was also found for pain (ß, −0.88; 95% CI, −1.18 to −0.57; P ≤ .001), perceived effect of treatment (ß, 1.40; 95% CI, 0.97 to 1.82; P ≤ .001), and HRQoL (ß, 0.11; 95% CI, 0.08 to 0.14; P ≤ .001). For the proportion of patients who met the MIC and poor disability cutoffs, results also favored the SPLIT program. Sensitivity analysis showed similar results when considering only participants recruited during the implementation plan (Supplemental Table 1 and Supplemental Table 2). Exploratory analysis suggested that whereas for the first cohort, improvements appeared to have been inferior for patients with high risk of poor disability; for the second cohort, benefits of the SPLIT program appeared consistent over the distinct risk subgroups (Supplemental Table 3 and Supplemental Table 4).
Primary and Secondary Outcomes Over the 6-Month Follow-Up
Effect Estimates for Comparison of Cohorts (SPLIT vs Usual Care) for Primary and Secondary Outcomes Over the 6-Month Follow-Up
DISCUSSION
In the present before-and-after study, implementation of the SPLIT program for patients with LBP in primary care was associated with greater improvements in back-related disability, pain, perceived effect of treatment, and HRQoL compared with UC over a 6-month follow-up period.
Our results suggest a superior benefit of the SPLIT program vs UC compared to previous studies testing a similar stratified approach in primary care in different countries.12,14,16-20 Interestingly, our findings occurred even considering the predominant proportion of patients with low risk of poor disability within our sample.
The described benefits challenge the idea that the stratified approach is only successful within the context of UK primary care, where it was initially developed. This was implied after no differences between interventions were found between the United States18 and Denmark.19 Specificities of health context and methodologic options might help explain these results. In the United States, despite the comprehensiveness of the implementation plan,48 only one-half of the FPs used the SBST, and of those who did, knowledge about each patient’s risk did not change the referrals for matched treatments.18 This might be justified by (1) the predominancy of the training program on general concepts and use of SBST and less on the matched treatments for each risk subgroup, (2) the lack of a clear clinical pathway, especially for medium- and high-risk patients, because distinct clinicians could complete the SBST and treat patients accordingly with numerous treatment options (eg, physical therapy, acupuncture, chiropractic), or (3) the absence of a monitoring program to audit/provide feedback for clinicians responsible for treating patients.48 In the present study, both risk stratification and treatment procedures were the PTs’ responsibilities alone. In addition, the standardization and structure of the training and mentoring program for these clinicians, along with their lack of experience and confidence in managing musculoskeletal disorders,31 might have contributed to the lack of belief regarding alternative interventions and thus potentiated the fidelity to the matched treatments. The average number of physical therapy sessions provided to each risk subgroup, which was aligned with the guidance given to participating PTs, might be indicative of this fidelity to matched treatments.
To the best of our knowledge, this is the first study aiming to implement a stratified approach for LBP in the routine clinical practice of Portuguese primary care. We observed high retention during follow-up. Notwithstanding, several limitations need to be considered. First, this was not a randomized controlled trial, and thus several factors could explain changes in health-related outcomes for the SPLIT cohort. The complexity of the Portuguese health context and the great probability of contamination of the randomization procedure (even with a cluster design given the decreased number of PTs and the same working region), as well as our intention of mirroring routine clinical practice, explain our option for a before-and-after design. Second, the recruitment of participants was based soley on the participating FPs; therefore, selection bias cannot be excluded. This is especially important considering the baseline imbalances observed between cohorts. Despite the adjustments of the effect estimates for these imbalances, results should be interpreted with caution. Measurement (ie, different assessors between cohorts) and performance bias (ie, more attention given to participants in the SPLIT cohort) also need to be considered. Third, approximately 41% of the referred participants in the SPLIT cohort were excluded, mainly owing to patients’ lack of interest in participating or dropouts during the treatment program. Despite anticipation of this barrier,31 this might represent proof of selection bias. Fourth, the COVID-19 pandemic period might have had unknown effects on our findings. However, the results of our sensitivity analysis showing a limited effect of this deviation from protocol cannot be ignored. Finally, the change in PTs’ behavior was not measured, and we cannot confirm they applied the knowledge that the training and mentoring program entailed.
In the future, investigation of the economic effect of the SPLIT program should be prioritized. The constraints of the COVID-19 pandemic largely hindered access to LBP-related medical records, preventing this analysis.
In conclusion, the implementation of a stratified approach for LBP in Portuguese primary care might promote substantial benefits for patients, supporting the recommendation that further development and implementation of evidence-based managing approaches within local health systems should be prioritized.4
Acknowledgments
The team of authors wishes to acknowledge the invaluable support and cooperation of the Regional Health Administration of Lisbon and Tagus Valley (ARS-LVT); clinical boards of the Arrábida Health Center Group (ACeS Arrábida); and the family physicians and physical therapists of the participating health units, their staff, and the patients involved in this study.
Footnotes
Conflicts of interest: authors report none.
Funding support: This study was funded by the Fundação Ciência e Tecnologia, IP national support via the Comprehensive Health Research Centre (CHRC) (UIDP/04923/2020). Luís Antunes Gomes is supported by the same institution under an individual PhD grant (SFRH/BD/145636/2019).
Previous presentations: Gomes LA, Fernandes R, Caeiro C, et al. CO181 - The SPLIT Stratified Model of Care for Low Back Pain – Results From an Implementation Study in the Portuguese Context Of Primary Healthcare, paper presented at the XXIII Congresso Português de Reumatologia (Portuguese Congress of Rheumatology); October 13-16, 2021; Albufeira, Portugal.
Trial registration: ClinicalTrials.gov: NCT04046874 (August 6, 2019). Retrospectively registered.
- Received for publication April 18, 2023.
- Revision received January 29, 2024.
- Accepted for publication February 2, 2024.
- © 2024 Annals of Family Medicine, Inc.