Abstract
PURPOSE This study aimed to identify the demographic, psychiatric, and attitudinal predictors of treatment adherence during the maintenance phase of antidepressant treatment, ie, after symptoms and regimen are stabilized.
METHODS We surveyed 81 primary care patients given maintenance antidepressant medications regarding general adherence, recent missed doses, depression and treatment features, medication beliefs (necessity, concerns, harmfulness, and overprescription), and other variables. Additional data were collected from medical and payer records.
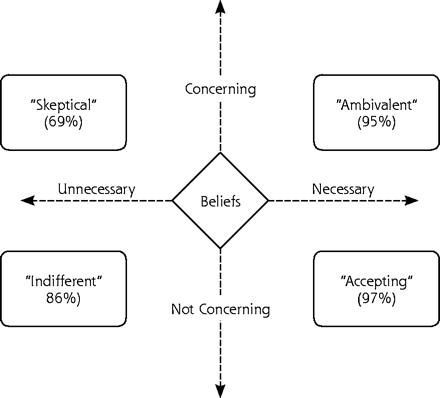
RESULTS Median treatment duration was 75 weeks. Adherence and beliefs were broadly dispersed and unrelated to treatment duration and type, physical functioning, and demographics. Multivariate analysis adjusting for social desirability, depression severity, and treatment duration indicated that an antidepressant-specific “necessity-minus-concerns” composite was strongly associated with both adherence outcomes. Specifically, adherence was highest when necessity exceeded concerns and lowest when concerns exceeded necessity. We crossed these 2 dimensions to characterize 4 patient attitudes toward antidepressants: skepticism, indifference, ambivalence, and acceptance.
CONCLUSIONS Patients given maintenance antidepressants vary widely in adherence. This variation is primarily explained by the balance between their perceptions of need and harmfulness of antidepressant medication, in that adherence is lowest when perceived harm exceeds perceived need, and highest when perceived need exceeds perceived harm. We speculate on ways to tailor adherence strategies to patient beliefs. Subsequent research should determine whether patients’ perceptions about medication predict depression outcomes, can be used to improve clinical management, and respond to behavioral intervention.
- Treatment refusal
- patient nonadherence
- depression/drug therapy
- health beliefs
- attitude to health
- patient compliance
INTRODUCTION
Major depressive disorder is associated with enormous personal suffering for affected patients, great distress for their family and friends, and major economic and societal costs.1,2 Antidepressant medication reduces depressive symptoms,3–,5 and its premature discontinuation increases the risk for relapse.6 Treatment guidelines recommend continuing antidepressant medication for at least 8 months after symptom remission and regimen stabilization, and this period is referred to as the maintenance phase of treatment.3,7 Nevertheless, between 30% and 83% of patients who begin antidepressants discontinue treatment prematurely.6,8–,10 Among primary care patients who filled a new prescription for a tricyclic antidepressant, 21% discontinued medication within 2 weeks of initially filling the prescription, an additional 3% to 10% discontinued every 2 weeks, until only about one half still took the medication at 4 months.9
Although most interventions to improve depression outcomes emphasize broad systems strategies, such as case identification and intensified follow-up, outcomes ultimately depend upon patients’ willingness to take antidepressants. In turn, willingness to take antidepressants is tightly linked to beliefs about medication. For example, patients report that treatment effectiveness and barriers are among the most critical aspects of depression care.11 Data also indicate that pretreatment perceptions of the benefits of and barriers to antidepressants predict initial medication adherence, and that primary care patients frequently attribute their early discontinuation to their perception that they do not need an antidepressant.9
The brief timelines of these existing studies, however, make it difficult to extrapolate their findings to the maintenance phase of treatment. This research gap is important, because adherence during treatment initiation in the early acute phase is probably governed by mechanisms different from those that govern maintenance-phase adherence. Patients on maintenance antidepressants are by definition less depressed than those in the acute phase; therefore, their treatment perceptions are probably less negatively biased. Moreover, the existing studies (reviewed above) collectively suggest that early discontinuation is related to both side effects and perceiving medication as ineffective, factors that seem unlikely to influence established antidepressant users whose initial side effects have subsided or who have experienced a treatment response. Instead, we suspect that long-term adherence may gradually diminish as clinically improved patients begin to conclude that they no longer need medication or become less willing to continue tolerating previously acceptable medication problems, such as sexual side effects. Maintenance-phase patients may also be relatively more affected by fears of potential long-term cumulative or insidious adverse effects, such as personality change, addiction, or toxicity.
Existing studies of beliefs about antidepressants also have several methodologic limitations. All previous studies used single ad hoc items rather than measures of validated beliefs, none used multiple adherence indices, and none adjusted for self-report biases, such as social desirability. Some studies aggregated patient-initiated discontinuation together with physician-initiated discontinuation even though these events are different. Many studies disregard meaningful variance by reducing adherence into a binary construct (present vs absent), although adherence is clearly a continuous gradient. All previous studies include tricyclic antidepressants, which have been displaced as first-line approaches by more contemporary agents that tend to have fewer side effects. In the present study, therefore, we endeavored to overcome common methodologic limitations by using multiple validated measures of adherence and beliefs, treating adherence as a continuum and adjusting for social desirability bias.
Research on beliefs about antidepressants has been exploratory and generally atheoretical. The present study was guided by Horne’s theoretical model of medication representations,12 which distinguishes between beliefs surrounding medication in general and beliefs surrounding the specific prescribed medication. This model breaks down medication-specific beliefs into 2 constructs—perceived need for the medication (necessity) and the perceived potential for the medication to cause problems (concerns)—and posits that adherence is determined by the balance between these 2 constructs. We thus hypothesized that adherence is highest for patients whose perceived need exceeds their concerns, and lowest under the reverse conditions. We did not expect beliefs about general medications to predict maintenance-phase adherence, because they seem more relevant to early adherence during treatment initiation rather than eventual adherence after patients are well established on the regimen.
METHODS
Study Participants
Patients for the study were recruited from a 12-physician family medicine clinic affiliated with the University of Michigan Health System. Inclusion criteria were age 18 years or older and medical chart evidence of 12 or more weeks of continued prescriptions for any first-line antidepressant* prescribed at a stable dosage specifically for the purpose of treating depression. We did not solicit patients who were on a mood stabilizer, whose medical record indicated cognitive impairment, or who were otherwise deemed inappropriate for the study by their primary care physician. Patients responding were typically women in their early 40s, who had a partial college or 2-year education degree and no serious medical condition (Table 1⇓).
Descriptive Statistics (n = 81)
Procedures
Under a protocol approved by the Institutional Review Board, study-eligible patients were identified by scanning the problem list of a clinical database13 that physicians verify and update at each clinical encounter. Eligible patients were mailed a study invitation signed by their primary care physician, an informed consent document, the self-report instruments, and a prepaid return envelope. Patients were paid $10 for participating in the survey.
Measures
We assessed 2 separate aspects of adherence. Recent percentage of adherence was measured using the first 3 items from the Brief Medication Questionnaire,14 which was previously validated against electronic medication-event monitoring data. Participants indicated the number of days in the past 2 weeks that they took their antidepressant as directed by their physician, the dosage they took, and the number of doses they took per day. Reported number of days of treatment adherence was then divided by 14 and multiplied by 100 to reflect recent percentage of adherence. Second, general adherence was assessed using a well-validated instrument developed by Morisky et al15 that elicits information about the presence or absence of various forms of nonadherence (eg, “In general, are you careless at times about taking your antidepressant medication?”).
Medication beliefs were measured with the Beliefs About Medication scale of Horne et al,16 which assesses medication-specific and general beliefs using 5-point Likert agreement-disagreement scales. The 2 medication-specific scales (necessity and concerns) consist of 5 items each (eg, “My current mental health depends upon my antidepressant medication” [necessity], and “I sometimes worry about becoming too dependent upon my antidepressant” [concerns]), whereas the 2 general scales (overprescription and harmfulness) have 4 items each concerning medications in general.
Depressive symptom severity was measured with the Patient Health Questionnaire (PHQ-9).17,18 This well-validated and reliable self-report version of the Primary Care Evaluation of Mental Disorders (PRIME-MD) mood module elicits 2-week retrospective frequency ratings (0 = not at all, through 3 = nearly every day) of 9 depressive symptoms; summed totals exceeding 9 indicate probable major depressive disorder.
Physical functioning was assessed with the physical component summary of the widely used Medical Outcomes Study SF-12 Health Survey (QualityMetric, Inc, Lincoln, RI). Social desirability bias, ie, the tendency to answer questionnaires in the socially acceptable direction, was assessed with the 20-item version of the Marlowe-Crowne Social Desirability scale.19 Antidepressant side effects were assessed with 3 items covering the frequency, intensity, and associated impairment of perceived side effects, each rated on positively keyed 7-point scales. The presence of 14 chronic medical conditions was assessed through self-report and verified by chart review, and demographic features were assessed with self-report items.
Data Analysis
Data were analyzed using SPSS 11.02 for Mac OS X (SPSS, Inc, Chicago. Ill). Descriptive statistics were calculated to characterize the sample and evaluate central tendency, variability, and distribution for key variables. To facilitate interpretation, necessity and concerns scores were Ζ-transformed, after which Ζ (concerns) was subtracted from the Ζ (necessity) to yield a necessity-minus-concerns composite score. The same procedure was applied to combine overprescription (negatively keyed) and harmfulness scores. Continuous variables with skewness indices of 0.4 or more were converted to ranks or log-transformed before data analysis. Missing data were not imputed. Zero-order associations were evaluated using χ2 statistics for categorical associations, Student t tests for categorical by continuous associations, and Pearson correlations for continuous associations. To adjust for multiple dependent variables, the study hypothesis was evaluated using general linear model multivariate analysis of variance (MANOVA), with the 2 adherence indices as the multiple dependent variables. Univariate ANOVAs were conducted to follow up significant multivariate effects, with the Bonferroni-corrected type I error rate set at less than .025 (2-tailed) for the 2 dependent variables. We modeled random effects terms to estimate intraclass correlation that could potentially arise from patients being clustered within physicians.
RESULTS
Response Rate
Of 171 mailed research solicitations, 95 (56%) were returned, 14 of which were incomplete, resulting in 81 analyzable cases. There were no significant differences between participants and nonparticipants in age, sex, or ethnicity (all P <.28).
Descriptive Statistics
Depressive symptom severity (Table 1⇑) ranged from 0 to 25 (interquartile range = 8), had a median of 8, and was mildly skewed (skewness = +0.55). Based upon PHQ-9 cutoffs, slightly less than one third of the study sample could be classified as currently in remission, with the remainder of the sample split evenly between having mild symptoms and having at least moderate symptoms. Patients had been on their antidepressant for a median of 75 weeks. Recent percentage of adherence ranged from 0% to 100%, with a mean of 85% (± 30.0%). General adherence scores also ranged fully from 0 to 3, and averaged 0.89 (± 0.96). Necessity and concerns scores were not significantly interrelated (r = −.08, P = .46), whereas overprescription and harmfulness scores were moderately intercorrelated (r = .41, P <.001).
Validity and Confound Checks
Because the validity of self-reported adherence data is frequently questioned, we compared self-reported general adherence scores with 3-month medication possession, computed from pharmacy refill data shared by a single health maintenance organization covering approximately 40% of our sample. There was 72% agreement (P = .015) between dichotomous classifications based upon general adherence and the medication possession ratio. Because treatment and depression duration were both free to vary, we tested whether either variable explained adherence or other dimensions. Student t tests and χ2 analyses, however, showed no significant differences in medication adherence between groups based on depression severity, medication beliefs, adherence, medical comorbidity, age, or sex; nor did adherence differ significantly by whether a patient saw a mental health professional. Finally, there appeared to be minimal intraclass correlation resulting from patients being nested within physician clusters (ρ= .03). Given that this statistic fell squarely within Hox’s “small” range of magnitude,20 and because we have a relatively high number (10) of physician clusters with a median of only 8 patients each, we did not adjust data analyses for dependency within clusters.
Hypothesis Testing
MANOVA was used to analyze general and recent adherence simultaneously as dependent variables (Table 2⇓). The additional variables considered for inclusion in the model were demographics (age, sex, and educational level), depression severity and duration, and social desirability bias. To preserve degrees of freedom, the 3 demographic covariates were dropped from the model because they had no significant multivariate effects (all P >.23); we retained depression characteristics and social desirability because of their theoretical relevance to adherence. As can be seen in Table 2⇓, only the medication-specific necessity-minus-concerns composite score had a significant multivariate association with adherence (P <.001), which univariate ANOVA on follow-up indicated was due to its association with both general adherence (ie, Morisky score, P <.001) and recent adherence (P = .001). There were no other significant multivariate associations, nor did the pattern of statistical significance change when social desirability and the depression variables were dropped from the model.
Results of MANOVA Analysis of Adherence
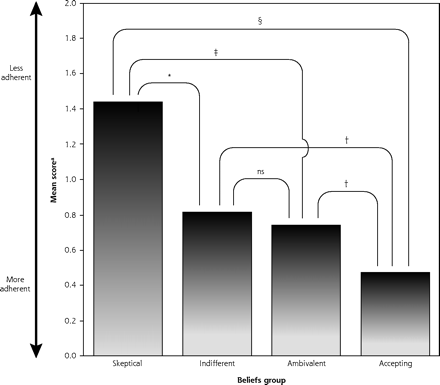
To translate the necessity-concerns differential into a categorical perspective that might be more useful to clinicians, we split at the median both belief dimensions to create 4 antidepressant attitude groups: (1) skeptical (low necessity, high concerns; n = 24), (2) ambivalent (high necessity, high concerns; n = 18), (3) indifferent (low necessity, low concerns; n = 19), and (4) accepting (high necessity, high concerns; n = 20). Figure 1⇓ illustrates how these groups were derived. A series of Student t tests indicated that general adherence varied significantly by group (Figure 2⇓). Specifically, the antidepressant-accepting group had significantly lower Morisky scores than each of the other 3 groups (all P <.05). Second, patients who were skeptical about antidepressant medications were significantly less adherent to treatment than both ambivalent and accepting patients (both P <.05), although their difference from indifferent patients fell just short of statistical significance (P = .057). The pattern of findings was similar for recent percentage of adherence scores (Figure 1⇓), with the exception that ambivalent patients also did not differ significantly from accepting patients.
Antidepressant belief groups (recent percentage of adherence in parentheses).
Morisky adherence scores by beliefs group. Note: Higher scores indicate lesser adherence.
ns = nonsignificant.
* P = .057
† P = <.05
‡ P = <.01
§ P = <.001
DISCUSSION
To summarize, we found that adherence to maintenance antidepressant therapy averaged 85%, varied broadly, and varied as predicted according to patients’ beliefs about the medications. Of the many factors that we analyzed or adjusted; including demographics, social desirability, and depression characteristics; beliefs about the necessity for and concerns about antidepressant medications were the only variables that accounted for adherence. Specifically, both recent and general adherence were highest among patients whose perceived need for medication exceeded their concerns about taking medication, and lowest for those whose concerns about taking medication exceeded the perceived need. These 2 dimensions can be reduced into an intuitive composite score that reflects the balance between perceived necessity and concerns.
Interestingly, necessity and concerns were empirically orthogonal in our sample of patients on long-term antidepressant therapy. That is, antidepressant concerns were no more likely among patients who believed that they needed their medication than among those who saw it as unnecessary. Similarly, viewing one’s antidepressant as unnecessary was not associated with also viewing it as unsafe. Even more interesting, both beliefs were independent predictors of adherence. Taken together, this pattern of findings challenges the seemingly common notion that patients’ concerns about medication reflect their underlying disagreement with the treatment plan, and that a perceived need for medication mitigates medication-related concerns. Subsequent research ought to explore the empirical and pragmatic utility of comparing beliefs within patients, which has the advantage of operating at the patient level where decision making plays out. Another advantage of this approach is that it helps control for individual biases in how study participants use rating scales, ie, some patients tend to use extreme ratings whereas others tend to use more moderate or neutral ratings.
Although our cross-sectional data cannot support causal conclusions, several major theoretical models (eg, Social-Cognitive Theory, Health Beliefs Model, Commonsense/Self-Regulation Model) emphasize that beliefs determine behavior. Of these traditions, the Health Beliefs Model makes the most specific statements about treatment beliefs through its effectiveness and barriers constructs. The extension of Leventhal’s Commonsense Model21 by Horne et al,12 however, is the most specific model in regard to medication, postulating that adherence is governed by patients’ mental representations of whether they require medication and whether it will cause problems. Our findings specifically support the medication-specific dimensions of the medication representations model and complement a growing literature on medication beliefs across several other conditions, including hypertension,22 human immunodeficiency virus infection and acquired immunodeficiency syndrome,23 asthma, renal failure, heart disease, and cancer.16 In contrast, we did not find that general beliefs about medications did not predict maintenance adherence, nor did we expect to find this association. We are currently analyzing medication beliefs in 2 additional study groups in which we expect general beliefs to play a more central role: patients initiating antidepressants, and persons taking over-the-counter St John’s wort for depression.
According to most cognitive models, beliefs usually remain stable in the face of repeated experiences that specifically disconfirm them. Thus, data such as ours could lead to the development of new belief-based adherence promotion strategies for clinicians as well as for those conducting clinical trials. For example, patients who are indifferent about antidepressant medications (Figure 1⇑, lower left quadrant) might be most influenced by pragmatic factors, such as ease of administration and cost; or by social factors, such as patient-physician relationship, life stress, stigma, and social support. Perhaps these patients’ adherence behavior would be enhanced by an aggressive “built for speed” regimen that is specifically tailored to achieve rapid and full effects. These patients might also benefit from education about symptom course, medication response lag, the rationale of maintenance treatment, and the link between early discontinuation and subsequent decline.
In contrast, patients who are ambivalent about antidepressant medications might be more motivated to minimize medication problems than to achieve rapid and complete symptom relief. Thus, their adherence might be enhanced by a more conservative “built for comfort” regimen that minimizes side effects. They might also benefit if clinicians proactively identify and correct any patient misunderstandings about medications, emphasize the transient or reversible nature of most side effects, and respond rapidly to any patient-expressed medication concerns.
Finally, patients who are skeptical of antidepressant medications will probably not be motivated to take antidepressants until either their perceived need increases, concerns diminish, or symptoms worsen. Behavioral strategies might initially affect their beliefs but not their adherence behavior, and sustained effort will be needed to achieve adequate medication trials. It may be beneficial to explore whether they view themselves as affected by nonbiological depression or consider their condition as otherwise misdiagnosed, whether they have a history of insufficient or overaggressive treatment or they have cultural beliefs about medication. Although sometimes their multiple treatment barriers can be resolved, to do so within the constraints of the primary care setting is particularly challenging.
Given certain study limitations, our findings should be taken as preliminary and not necessarily generalizable to all patient populations. Our study does not apply to patients who discontinue drug therapy during treatment initiation (a group that is already well-researched). Even though we did recruit some patients who did not adhere to their treatment regimen, self-selection probably biased respondents toward a more adherent study sample. We did not control the study for the length of depression or its treatment, although each proved unrelated to adherence. A more ethnically diverse sample would have increased the sensitivity of our study to cultural effects upon beliefs, and the findings might not apply to patients who seek specialized mental health treatment.
Our cross-sectional data cannot prove that beliefs play a causal role. Although we were able to rule out confounding by depression variables, medication side effects, social desirability bias, medical comorbidity, and demographic features, adherence or beliefs may actually be governed by some unmeasured factor, such as a patient-physician relationship, stigma, or memory problems. Finally, we could not confirm that patients met criteria for a depressive syndrome when their treatment episode began. Even so, patients with physician-recognized depression tend to have greater symptom severity and recurrence than do patients whose criteria-defined depression is not recognized by their physician.24
In closing, our findings raise a variety of testable implications for predicting treatment adherence and developing strategies tailored to enhance it. Future research ought to examine whether attitudes about antidepressant medications prospectively predict treatment adherence and outcome. If so, subsequent studies ought to determine whether depression outcomes improve when regimens are tailored to patients’ beliefs, and explore the generality of our proposed attitudinal prototypes apart from antidepressant medication regimens.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views of the University of Michigan or the Blue Cross Blue Shield Foundation of Michigan.
Acknowledgments
Erin Rickard, Andrea Plaut, and Beth Duncan provided editorial feedback and helpful comments on earlier drafts of this manuscript, and Dr. Robert Horne provided scientific feedback on the penultimate draft.
Footnotes
-
↵* Either bupropion (Wellbutrin), citalopram (Celexa), escitalopram (Lexapro), fluoxetine (Prozac but not weekly formulation), fluvoxamine (Luvox), mirtazapine (Remeron), nefazodone (Serzone), paroxetine (Paxil), sertraline (Zoloft), or venlafaxine (Effexor).
-
Conflicts of interest: none reported
-
Funding support: This research was supported by a grant from the Blue Cross Blue Shield Foundation of Michigan.
- Received for publication March 9, 2004.
- Revision received July 1, 2004.
- Accepted for publication August 30, 2004.
- © 2005 Annals of Family Medicine, Inc.