Abstract
PURPOSE The US Preventive Services Task Force (USPSTF) is an independent body that makes evidence-based recommendations regarding preventive services to improve health for people nationwide. Here, we summarize current USPSTF methods, describe how methods are evolving to address preventive health equity, and define evidence gaps for future research.
METHODS We summarize current USPSTF methods as well as ongoing methods development.
RESULTS The USPSTF prioritizes topics on the basis of disease burden, extent of new evidence, and whether the service can be provided in primary care and going forward will increasingly consider health equity. Analytic frameworks specify the key questions and linkages connecting the preventive service to health outcomes. Contextual questions provide information on natural history, current practice, health outcomes in high-risk groups, and health equity. The USPSTF assigns a level of certainty to the estimate of net benefit of a preventive service (high, moderate, or low). The magnitude of net benefit is also judged (substantial, moderate, small, or zero/negative). The USPSTF uses these assessments to assign a letter grade from A (recommend) to D (recommend against). I statements are issued when evidence is insufficient.
CONCLUSIONS The USPSTF will continue to evolve its methods for simulation modeling and to use evidence to address conditions for which there are limited data for population groups who bear a disproportionate burden of disease. Additional pilot work is underway to better understand the relations of the social constructs of race, ethnicity, and gender with health outcomes to inform the development of a USPSTF health equity framework.
- preventive medicine
- clinical practice guidelines
- methodology
- health equity
INTRODUCTION
The US Preventive Services Task Force (USPSTF) is an independent body formed in 1984 to make evidence-based recommendations regarding preventive services including screening, behavioral counseling, and preventive drugs. The 16 members are appointed by the Director of the Agency for Healthcare Research and Quality and come from the fields of family medicine, internal medicine, nursing, obstetrics and gynecology, pediatrics, and preventive and behavioral medicine, with broad and deep expertise in preventive medicine and primary care. The USPSTF members disclose financial and nonfinancial interests. The USPSTF uses rigorous methods that include comprehensive systematic reviews addressing the benefits and harms of preventive services. The USPSTF’s recommendations are for primary care clinicians and asymptomatic patients. The objectives of this article are to describe the evolving methods of the USPSTF since prior overviews,1,2 to discuss ongoing refinement of our methods and the stakeholder engagement process to address prevention more equitably, and to review our recent efforts to better classify evidence gaps. Additional details on USPSTF methods are available in its Procedure Manual.3
Topic Nomination, Prioritization, and Updating
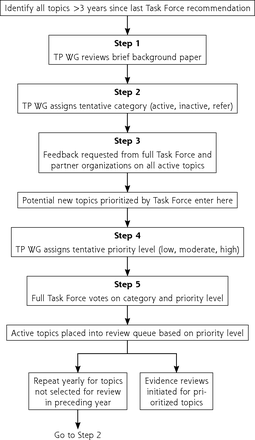
Recognizing the importance of diverse perspectives, any individual or group can nominate a new topic or request an update of an existing topic. The Topic Prioritization Workgroup, a subset of USPSTF members, reviews nominations to assess whether topics are in scope and focus on asymptomatic people and whether the service can be delivered or referred from primary care. The steps for topic nomination, prioritization, and updating are outlined in Figure 1. The Workgroup also reviews active topics 2 to 3 years after publication to determine whether to keep the topic active and whether the evidence on the preventive service can be updated via an expedited process. This prioritization process is informed by a background document produced by the USPSTF’s Scientific Resource Center, providing the prevalence of the condition and relevance to primary care and summarizing new evidence and in-process studies. To better address health equity, the USPSTF now gives greater emphasis to disease burden among groups, such as Black, Hispanic/Latino, Asian and Pacific Islander, and Indigenous people, during prioritization. In addition to the background document, a prioritization survey provided by the Agency for Healthcare Research and Quality (AHRQ) and completed by USPSTF members and partner organizations informs the queue of topics for the next 12 to18 months.
Steps in topic prioritization.
TP WG = Topic Prioritization Workgroup.
Development of the Research Plan
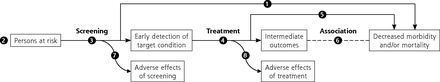
The first step in developing or updating a recommendation is a research plan comprised of an analytic framework, key questions, and inclusion and exclusion criteria specific to the key questions and contextual questions. In the draft research plan, the USPSTF now describes steps to address equity and study heterogeneity in a new section titled Approach to Assessing Health Equity and Variation in Evidence Across Populations. The plan is developed by an Evidence-Based Practice Center (EPC) in collaboration with the USPSTF and AHRQ. The analytic framework is a graphical representation of the evidence needed to connect the performance of a preventive service to a health outcome; it depicts the population under consideration, interventions, intermediate health outcomes, and final health outcomes, capturing both benefits and harms (Figure 2). Key questions articulate the chain of evidence needed to determine the net benefit of a preventive service. Contextual questions address other important considerations for the recommendation such as barriers to accessing interventions. The draft research plan is posted on the USPSTF website (uspreventiveservicestaskforce.org) for input.
Generic screening analytic framework.
Note: Numbers in figure correspond to key questions that are addressed by the systematic evidence review. For example, Key Question 1 relates to the direct evidence as to whether screening for a condition affects health outcomes that are important to patients. For more information about analytic frameworks, see the Task Force Procedure Manual.3
Systematic Evidence Review
Systematic reviews addressing key questions are conducted by EPCs and follow the rigorous methods of the AHRQ EPC program4 in addition to those of the USPSTF. These methods evolve to innovate best practices in evidence synthesis including evidence of the effect of preventive services among populations bearing a disproportionate burden of disease. The USPSTF considers randomized controlled trials and well-conducted systematic reviews and meta-analyses as methodologically strongest. Separate methods have been developed to conduct expedited reviews for topics suitable for reaffirmation.5
Because the USPSTF has many counseling topics, recommendations include a table describing the key intervention characteristics, which allows the USPSTF to provide information to help facilitate implementation.6 Although the systematic reviews focus on randomized controlled trials, nonrandomized studies with unbiased comparator groups may be included to address limitations in the trial evidence on the effectiveness or harms of any given preventive service. Finally, the USPSTF recognizes that improving the health of people nationwide necessitates improving the health of those who experience greater morbidity and mortality from the condition; therefore, the USPSTF continues to innovate methods to synthesize evidence for these populations7 and integrate this evidence into recommendations.8,9 This article provides additional details on our efforts to address health equity at different steps of our process.
Use of Simulation Modeling
The USPSTF commissions modeling studies10,11 when empiric data are sufficient to recommend a preventive service but important questions remain. For screening, the questions are typically regarding intervals for screening, starting and stopping ages, and the screening tests used.12-19 The USPSTF does not make recommendations on the basis of modeling alone without supporting empiric evidence. The USPSTF usually considers multiple models simultaneously.11 Because these collaborative models are developed independently, they use different assumptions and structures. When collaborative models yield consistent findings, they provide a robust basis for answering remaining questions.
The recommendation statement on screening for lung cancer shows how collaborative modeling can inform important aspects of a preventive service.18,19 Four independent Cancer Intervention and Surveillance Modeling Network models supplemented evidence from 2 trials showing a decrease in lung cancer mortality. These models evaluated how health outcomes varied with different start and stop ages, screening intervals, and smoking histories.18
Recommendation Development
Assessing Adequacy of Evidence
After the systematic review, the USPSTF assesses the adequacy of evidence for each key question on the basis of the body of evidence’s internal and external validity. The evidence’s adequacy to address linkage coherence across the analytic framework is also considered; that is, whether the body of evidence makes sense and fits together. The adequacy of evidence at the key question and linkage level is categorized as convincing, adequate, or inadequate. In assessing evidence adequacy, the following 6 questions are considered:
Do the studies have the appropriate research designs?
Are the studies of sufficient quality?
Are the results of the studies generalizable to the primary care population?
How many and how large are the studies?
How consistent are study results?
Are there additional factors that assist in drawing conclusions?
Assessing Magnitudes of Benefits and Harms
If the evidence is deemed convincing or adequate, the USPSTF then determines the magnitudes of benefits and harms of the preventive service. The magnitude of benefit describes the change in health outcomes that would be expected from providing vs not providing the service for a population. For example, screening interventions must lead to both earlier detection of the disease and better outcomes. Similarly, the magnitude of harm estimates the burden of harm that would be introduced by delivering the service. Given that preventive interventions are intended for asymptomatic individuals to mitigate future morbidity, the assessment of harms is critically important. The magnitude of benefit or harm is categorized as substantial, moderate, small, or zero. This estimate is based on effect sizes from studies as well as on the public health burden of the disease and the incidence, severity, and duration of outcomes. When evidence is limited, conceptual upper or lower bounds may be established by extrapolating from studies of different baseline risk populations or in settings other than primary care. Additional details are available in the USPSTF Procedure Manual.3
Assessing Coherence Linkage
Whenever possible, the USPSTF looks for direct evidence of benefit (Key Question 1 in Figure 2). Direct evidence is ideal for limiting bias, providing the greatest confidence. The USPSTF also examines the indirect evidence pathway, which connects the target population (far left of Figure 2) to improved health outcomes (far right of Figure 2) by linking Key Questions 3 to 8 (How accurate are screening tools? How well does treatment work? Can intermediate outcomes predict health outcomes? What are the harms of each step?). To make this linkage, the USPSTF looks at the coherence of the evidence, or how well the pieces fit together, and the applicability of the evidence to an asymptomatic primary care population. Compared to direct evidence, indirect evidence has a greater risk of bias.
Intermediate Outcomes
The Task Force defines a health outcome as a symptom, functional level, or condition that patients can feel or experience. Examples include functional status, quality of life, and mortality. Available studies on preventive services often do not report on health outcomes but instead on intermediate outcomes (Figure 2). Intermediate outcomes are pathologic, physiologic, social, or behavioral measures that a patient does not feel or experience. A preventive service might affect an intermediate outcome without improving health outcomes. The USPSTF has developed methods for considering the linkage between intermediate and health outcomes.20 When assessing linkage, the USPSTF looks for evidence showing a consistent relation between a change in an intermediate outcome and a change in health outcome. For example, regarding hepatitis C screening (Supplemental Figure 1), there was convincing evidence that newer antiviral regimens resulted in a sustained virologic response (an intermediate outcome) in a very high proportion of adults and adequate evidence of a consistent association between sustained virologic response and improved health outcomes (decreased all-cause and liver disease mortality). Given this linkage, the USPSTF issued a B recommendation for hepatitis C virus screening.21,22
Determining a Recommendation Grade
To make a recommendation, the USPSTF judges the certainty and magnitude of the net benefit (benefits minus harms) of the preventive service at the population level. Certainty, categorized as high, moderate, or low, is based on the quality of the evidence (see USPSTF Procedure Manual3 for more detail). Assessing certainty requires a synthesis of evidence across the analytic framework to judge whether the results observed would be expected when the intervention is delivered for primary care populations and how likely future research would change that assessment.
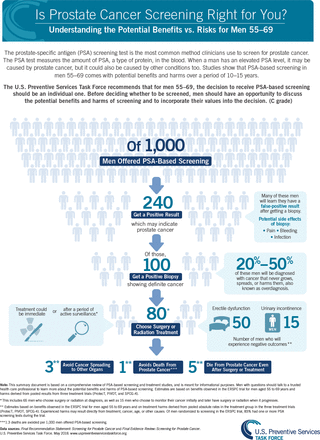
The magnitude of net benefit is categorized as substantial, moderate, small, zero, or negative. Assessing net benefit can be challenging because the metrics for benefits and harms often differ. For example, screening for prostate cancer with the prostate-specific antigen (PSA) test among men aged 55-69 years might prevent approximately 1 death per 1,000 men screened over a period of 10 years. Yet screening leads to many more men having a false-positive result and receiving a diagnosis of prostate cancer, leading to overdiagnosis and often overtreatment of cancers not destined to cause harm (Figure 3).23,24 Ultimately after extensive discussion, the USPSTF judged with moderate certainty that PSA screening provided a small net benefit. In addition, there might be insufficient evidence on net benefit to support an alternative screening strategy for populations at greater risk of the disease. For example, Black men have a lifetime probability of dying of prostate cancer of approximately 5% compared with 3% for White men. However, available evidence did not support a different PSA screening recommendation for Black men in part because of a lack of sufficient inclusion of Black men in the reviewed evidence. A call for more research was highlighted in the recommendation.
Estimates of the benefits and harms of PSA screening for prostate cancer.
PSA = prostate-specific antigen.
Note: Reprinted with permission from the Agency for Healthcare Research and Quality, acting on its own behalf and that of the US Preventive Services Task Force.
Table 1 shows how a letter grade is determined for a preventive service using the USPSTF estimates of certainty and net benefit. If certainty is low, an Insufficient Evidence or I statement is issued; the USPSTF does not use expert opinion to make recommendations when evidence is lacking.25,26
United States Preventive Services Task Force Recommendation Grade Grid: Magnitude and Certainty of Net Benefit
Communicating Recommendations
In its statements, the USPSTF describes the chain of evidence used to arrive at the recommendation in the Assessment of Magnitude of Net Benefit section and the Rationale Table. The evidence informing the recommendation is summarized in the Supporting Evidence section.
Understanding Grades
Table 2 provides a definition of each letter grade and corresponding suggestions for practice. For example, it was determined with moderate certainty that screening for hepatitis C virus infection in a population aged 18 to 79 years has substantial net benefit; therefore, a B grade was issued, meaning that clinicians should routinely recommend screening.21
How to Interpret Task Force Recommendation Grades
Practice Considerations
The Practice Considerations section provides clinicians a concise, streamlined summary of information needed to implement the recommendation.27 Companion materials may include infographics and office conversation guides.
Research Gaps
The USPSTF includes a Research Needs and Gaps section in its recommendations to communicate key research still needed.27 The USPSTF has become increasingly concerned about widespread inequities in preventive care such as those based on sex, gender, race, and ethnicity. The USPSTF is especially attuned to inequities in Black, Hispanic/Latino, Asian and Pacific Islander, and Indigenous populations that face systemic racism leading to greater risks of preventable diseases and a lower likelihood of receiving appropriate preventive services followed by diagnosis and treatment.8,9 The USPSTF continues to report research gaps addressing health inequities to the US Congress and research funders.
Stakeholder Engagement
The USPSTF values input from the public, specialists, and other stakeholders at every stage of the recommendation process. Via the USPSTF website (uspreventiveservicestaskforce.org), anyone can nominate topics and provide feedback on draft research plans, recommendation statements, and evidence reports. Every comment is considered by USPSTF members before finalization of documents. In addition, the USPSTF reaches out to stakeholder organizations directly and invites them to provide comments. All draft evidence reports are reviewed by experts in the field and USPSTF federal health partners; organizations with content expertise are also invited to nominate reviewers. The USPSTF continuously engages with federal and nonfederal partners via regular meetings. This feedback makes recommendations more understandable to clinicians and stakeholders.
Ongoing Methods Development
The USPSTF is dedicated to meeting the health needs of an increasingly diverse US population and recognizes the impact of social determinants on the delivery of preventive services. Given that many important population groups, particularly groups bearing a disproportionate burden of disease, are often not included in trials, the USPSTF continues to refine its methods to develop evidentiary rules (for nonrandomized studies, epidemiologic data, and modeling) and criteria for extrapolation to better address racial, ethnic, and gender disparities in the use of preventive services and in health outcomes.
The approach to addressing inequities is exemplified in the recent update of the USPSTF lung cancer screening recommendation.19 The updated recommendation was informed by new trial evidence and simulation modeling18 that allowed the USPSTF to identify the most efficient screening strategies, particularly among Black people, who have a greater burden of lung cancer. On the basis of simulation modeling, the 2021 recommendation, which decreased the starting age from 55 to 50 years and the smoking criterion from ≥30 to ≥20 pack-years, would increase the relative percentage of adults eligible for screening by 78% in non-Hispanic White persons, 107% in non-Hispanic Black persons, and 112% in Hispanic/Latino persons.
Potential approaches under consideration to address equity are the use of robust comparative cohort or interrupted time series studies with sufficient participant diversity to identify variations in net benefits by race, ethnicity, sex, gender, or social determinants of health. Additional analytic approaches, such as individual participant meta-analyses and modeling with race as an independent variable, may also be considered. Sex and gender of participants are not often clearly specified in studies of preventive services. The USPSTF is developing inclusive approaches to addressing sex and gender in recommendation development.28 Additional approaches include a taxonomy to categorize evidence gaps and inform future research addressing health inequities.29 As these changes crystalize, they will be reflected in updates to the Task Force Procedure Manual.3
There is a need to assess whether these approaches decrease any influence of systemic racism or sources of bias and inequity at each step of recommendation formation; for example, whether recommendations might create implementation barriers that disproportionately affect some population groups. This process will also inform the development of a health equity framework that aligns with these approaches. As part of future evidence reviews and as outlined in prior articles,8,9 the USPSTF will continue to pilot test the inclusion of evidence on variation in benefits and harms as well as implementation barriers by population groups.
CONCLUSIONS
The USPSTF is committed to recommending evidence-based clinical preventive services. Achieving this goal is critical to the health of a diverse US population. The USPSTF will continue to follow its traditional robust critical appraisal of the evidence while working to advance innovative methods to address conditions for which there are limited data for specific disproportionately affected groups. This evolution of methods will ensure that the USPSTF meets its mission of improving the health of all people nationwide.
Footnotes
Conflicts of interest: authors report none.
Read or post commentaries in response to this article.
Disclaimer: Drs Krist, Mangione, Owens, Davidson, Pbert, Barry, and Nicholson are current or recent members of the independent US Preventive Services Task Force (USPSTF), and they speak ex cathedra for the Task Force. Drs Mabry-Hernandez, Mills, Fan, and Wolff are staff at the Agency for Healthcare Research and Quality (AHRQ), and Dr Lin works with the USPSTF via a contract with the AHRQ. The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of the AHRQ. No statement in this manuscript should be taken as an official position of the AHRQ or the US Department of Health and Human Services.
Supplemental materials
- Received for publication July 11, 2022.
- Revision received October 24, 2022.
- Accepted for publication November 21, 2022.
- © 2023 Annals of Family Medicine, Inc.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.