Abstract
PURPOSE Acute sinusitis is a common condition in ambulatory care, where it is frequently treated with antibiotics, despite little evidence of their benefit. Intranasal corticosteroids might relieve symptoms; however, evidence for this benefit is currently unclear. We performed a systematic review and meta-analysis of the effects of intranasal corticosteroids on the symptoms of acute sinusitis.
METHODS We searched MEDLINE, EMBASE, the Cochrane Central register of Controlled Trials (CENTRAL), and Centre for Reviews and Dissemination databases until February 2011 for studies comparing intranasal corticosteroids with placebo in children or adults having clinical symptoms and signs of acute sinusitis or rhinosinusitis in ambulatory settings. We excluded chronic/allergic sinusitis. Two authors independently extracted data and assessed the studies’ methodologic quality.
RESULTS We included 6 studies having a total of 2,495 patients. In 5 studies, antibiotics were prescribed in addition to corticosteroids or placebo. Intranasal corticosteroids resulted in a significant, small increase in resolution of or improvement in symptoms at days 14 to 21 (risk difference [RD] = 0.08; 95% CI, 0.03–0.13). Analysis of individual symptom scores revealed most consistently significant benefits for facial pain and congestion. Subgroup analysis by time of reported outcomes showed a significant beneficial effect at 21 days (RD = 0.11; 95% CI, 0.06–0.17), but not at 14 to 15 days (RD = 0.05; 95% CI, −0.01 to 0.11). Meta-regression analysis of trials using different doses of mometasone furoate showed a significant dose-response relationship (P=.02).
CONCLUSIONS Intranasal corticosteroids offer a small therapeutic benefit in acute sinusitis, which may be greater with high doses and with courses of 21 days’ duration. Further trials are needed in antibiotic-naïve patients.
INTRODUCTION
Acute sinusitis is a common condition, affecting an estimated 31 million Americans annually.1 The majority of patients seen in primary care for acute sinusitis are prescribed antibiotics,2,3 despite evidence that they provide limited benefit.4–6 The effectiveness of other treatments such as decongestants and antihistamines is largely unknown.7,8 Corticosteroids are effective in reducing symptoms in other upper respiratory tract infections such as croup, sore throat, and infectious mononucleosis, and their anti-inflammatory effects may be helpful for reducing sinus congestion and facilitating drainage in sinusitis.9–12
Currently, it is not clear whether corticosteroids offer significant benefits for patients with acute sinusitis. In particular, there have been no good-quality double-blind randomized controlled trials (RCTs) examining oral corticosteroids in acute sinusitis, even though the oral route is favored for other upper respiratory tract infections. In terms of intranasal steroids, a Cochrane review of 4 RCTs showed a small beneficial effect on improvement of symptoms at 15 to 21 days; however, interpretation was limited by both high heterogeneity and differing outcome measures used in the primary studies.13 A recent large RCT found no difference between intranasal corticosteroids and placebo for sinusitis.14 This trial was not included in the recent Cochrane review.13
Given the conflicting evidence, there is a pressing clinical need to clarify whether intranasal corticosteroids should be prescribed for patients with acute sinusitis. Accordingly, we undertook a systematic review of the most recent evidence to attempt to resolve this question.
METHODS
Search Strategy and Selection
We included in our meta-analysis RCTs that compared intranasal corticosteroids with placebo in children or adults who had clinical symptoms and signs of acute sinusitis or rhinosinusitis, in outpatient (ambulatory) settings. We excluded studies examining patients with chronic/allergic sinusitis and studies performed exclusively in patient populations selected because of chronic underlying health conditions (eg, immunocompromised patients).
We searched MEDLINE, EMBASE, the Cochrane Library including the Cochrane Central register of Controlled Trials (CENTRAL), the Database of Reviews of Effectiveness (DARE), and the National Health Service Health Economics Database from the beginning of each database until February 2011 using a maximally sensitive strategy.15 Medical Subject Heading (MeSH) terms used included rhinosinusitis, sinusitis, and corticosteroids (including dexamethasone, beta-methasone, prednisone, and all variations of these terms) and viral and bacterial upper respiratory tract pathogens (full search strategy available from authors). Two authors independently reviewed the titles and abstracts of electronic searches, obtaining full-text articles to assess for relevance where necessary. Disagreements were resolved by discussion with a third author. We performed citation searches of all full-text papers retrieved.
Data Extraction and Quality Assessment
Two authors independently assessed the methodologic quality of studies. Quality was assessed using the criteria of allocation concealment, randomization, comparability of groups at baseline, blinding, treatment adherence, and percentage participation. Two authors independently extracted data using an extraction template. In both data extraction and quality assessment, disagreements were documented and resolved by discussion with a third author.
Primary outcomes included the proportion of participants with improvement or complete resolution of symptoms. Secondary outcomes included mean change in symptom scores over 0 to 21 days, adverse events, relapse rates, and days missed from school/work. Where necessary, we used Grab It XP Microsoft Excel software (http://www.datatrendsoftware.com) to extract data from figures.
Data Synthesis and Analysis
For pooled analysis of dichotomous outcomes, we calculated the risk difference (RD), 95% CI, and number needed to treat (NNT). For continuous variables, we used weighted mean difference and 95% CIs. We tested dose response by undertaking a post hoc subgroup analysis according to intranasal corticosteroid dosage. We used meta-regression in Stata (StataCorp, LP) to test subgroup interactions on the outcomes and the I2 statistic to measure the proportion of statistical heterogeneity for each outcome.16 Where no heterogeneity was present, we performed a fixed-effect meta-analysis. Where substantial heterogeneity was detected, we looked for the direction of effect and considered the reasons for this heterogeneity. Where applicable, we used a random-effects analysis or considered not pooling the outcomes and reporting the reasons for this.
RESULTS
Study Characteristics
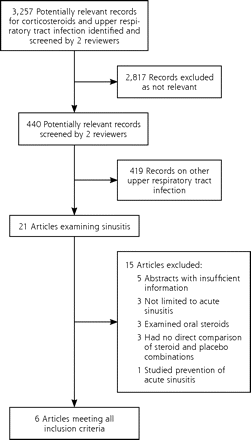
We identified 3,257 potentially relevant study records, of which 21 were relevant to acute sinusitis/rhinosinusitis (Figure 1). We excluded 15 of these studies for the following reasons: 5 were abstracts only with no full paper published or available from the authors, 3 were not limited to acute sinusitis, 3 examined oral steroids, 3 did not directly compare steroids and placebo, and 1 examined prevention of acute sinusitis.
Flow diagram of search results.
The characteristics of included studies are presented in Table 1. The 6 included studies randomized 2,495 patients recruited from outpatient otorhinolaryngology, emergency medicine, and general practice settings in 3 countries: the United States (4 studies), Turkey (1), and United Kingdom (1). The age range of participants varied: 12 years or older (3 studies); 16 years or older (1); 18 years or older (1); and 15 years or younger (1). The corticosteroids used were budesonide (2 studies), fluticasone propionate (1), and mometasone furoate (3). Two trials compared 2 different doses of mometasone furoate.17,18
Characteristics of Trials Included in the Meta-Analysis
In addition to intranasal corticosteroids, 5 trials14,18–21 prescribed antibiotics (amoxicillin, co-amoxiclav, or cefuroxime) to patients in both groups. One of these trials19 prescribed intranasal xylometazoline hydrochloride to all participants before administration of the study spray for the first 3 days. Two trials reported outcomes based on computed tomography scans of sinuses.18,21
All 6 included studies demonstrated adequate allocation concealment, blinding, percentage participation, and comparability of groups both at baseline and in provision of care apart from the intervention; however, 3 studies did not report the method of randomization (Table 2). We therefore performed a sensitivity analysis excluding these studies.
Methodologic Quality of Included Studies
Resolution or Improvement of Symptoms at Days 14 to 21
In 5 RCTs14,17,18,20,21 that assessed resolution or improvement of symptoms at days 14 to 21, intranasal steroids had a modest clinical beneficial effect, with an RD of 0.08 (95% CI, 0.03–0.13; P = .004; I2 = 47%) and an NNT of 13 (95% CI, 8–33). This overall result was similar even with the removal of the 2 trials of lower quality,18,21 with an RD of 0.07 (95% CI, 0.01–0.12; P = .02; I2 = 43%). Given that both analyses showed heterogeneity, however, we performed subgroup analyses on outcome timing and on dosage.
Outcome Timing
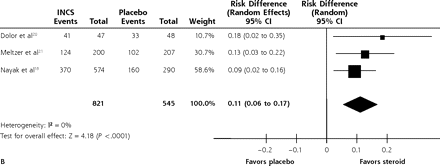
Combining the 3 studies reporting this outcome at 14 to 15 days14,17,20 showed no significant effect of intranasal corticosteroids, with an RD of 0.05 (95% CI, −0.01 to 0.11; P = .13; I2 = 22%) (Figure 2A). In contrast, combining the 3 studies that reported resolution or improvement at 21 days18,20,21 showed that intranasal corticosteroids had a significant beneficial effect with no heterogeneity, with an RD of 0.11 (95% CI, 0.06–0.17; P <.001; I2 = 0) and an NNT of 9 (95% CI, 6–17) (Figure 2B).
Effect of intranasal steroids on resolution of symptoms of acute sinusitis at (A) 14 to 15 days and (B) 21 days.
INCS=intranasal steroid.
In the 2 trials14,20 that reported the proportion of participants with persistent symptoms at 10 days after onset of treatment, there was no benefit of intranasal corticosteroids, with an RD of 0.06 (95% CI, −0.09 to 0.22; P = .41; I2 = 47%). Two trials reported a small but significant 7% absolute improvement in physician evaluation scores at 21 days in patients receiving intranasal steroids vs placebo (Nayak et al18: 61% vs 53%, P = .006; Meltzer et al21: 68% vs 61%, P <.01).
Dose-Dependent Benefit
Three trials using mometasone furoate nasal spray17,18,21 showed a significant effect on symptom resolution or improvement at 15 to 21 days, with an RD of 0.08 (95% CI, 0.01–0.14; P = .02; I2 = 62%) and an NNT of 13 (95% CI, 7–100). The daily dose of mometasone furoate ranged in these 3 trials from 200 μg to 800 μg. We therefore investigated the high heterogeneity by performing subgroup analysis by dose. As depicted in Figure 3, meta-regression analysis of mometasone furoate dose and symptom resolution showed a significant dose-response relationship (P = .02), with the effect size increasing with dose. For patients receiving 800 μg of mometasone furoate nasal spray daily, the RD was 0.12 (95% CI, 0.06–0.18; P = .0002) and the NNT was 8 (95% CI, 6–17); for those receiving 400 μg daily, the RD was 0.07 (95% CI, 0.03–0.11; P = .001) and the NNT was 14 (95% CI, 9–33).
Dose-response relationship of mometasone furoate and likelihood of symptom resolution.
Individual Symptom Scores
Three RCTs reported individual symptom scores in 5 groups of patients who received different doses of mometasone furoate compared with placebo17,18,21 For each group, the symptoms of facial pain, nasal congestion, headache, rhinorrhea, postnasal drip, and cough were reported on a scale of 0 (none) to 3 (severe) at baseline and averaged across the first 15 days of therapy (see the Supplemental Appendix at http://www.annfammed.org/content/10/3/241/suppl/DC1 for full data). Compared with their counterparts who received placebo, patients who received intranasal corticosteroids in these 3 trials reported significantly greater improvement in facial pain (3 of the trials), congestion (3), rhinorrhea (2), headache (1), and postnasal drip (1) (all P <.05).
Adverse Events
One trial reported that no adverse events occurred with steroid therapy19; 2 trials reported no serious adverse events in either group14,20; and the remaining trials reported that adverse events were mainly mild or moderate in severity.17,18,21 Meta-analysis demonstrated no significant differences in the rate of overall adverse events between patients taking intranasal corticosteroids (299 of 1,299, or 23%) and placebo (181 of 797, also 23%) (P = .83). Common adverse events were headache (noted in 2%–8% of patients, 4 studies), epistaxis (3%–7%, 4 studies), nasal irritation (1%–2%, 3 studies), and pharyngitis (2%–4%, 3 studies). Meta-analysis of the rate of occurrence of these outcomes revealed no significant differences between steroid and placebo groups.
DISCUSSION
Key Findings
This systematic review demonstrates that intranasal corticosteroids offer a small but significant symptomatic benefit in acute sinusitis. This effect is most marked when patients are given longer durations of treatment (21 days) and higher doses of the medication. Our analysis of individual symptom scores suggests that facial pain and nasal congestion may be most responsive to intranasal corticosteroids. In our main analysis, we found that whereas 66% of patients would experience improvement or resolution of symptoms at 14 to 21 days using placebo, an additional 7% of patients would achieve this outcome with corticosteroids, equating to an NNT of 13.
This 7% gain is a relatively small increase in the context of a self-limiting condition, and this clinical benefit must be set against potential harms and economic implications. Our included trials reported no serious adverse events associated with intranasal corticosteroid use and no increase in frequency of nonserious adverse events compared with placebo. Other potential harms might include effects from systemic absorption; however, the single included trial addressing this outcome found no clinically relevant changes in the hypothalamic-pituitary-adrenal axis,20 and 2 recent reviews found no evidence of suppression of this axis or of growth suppression with intranasal corticosteroids.22,23
Only 1 included trial assessed the potential benefit of intranasal corticosteroids for work and quality of life outcomes in acute sinusitis.20 In this trial, the corticosteroid group had a significantly higher subjective level of work performance (median, 100% vs 90% for placebo); however, there were no differences in work attendance or changes in quality of life as measured by the 20-item Sino-Nasal Outcome Test (SNOT-20)24 and the 12-Item Short Form Health Survey.25 An individual patient with acute sinusitis may therefore experience negligible adverse effects of intranasal corticosteroids in return for a small increase in likelihood of earlier resolution. This may be an acceptable trade-off for some patients. The therapeutic benefit at the population level is currently unclear.
Our subgroup analysis suggests the benefit of intranasal corticosteroids is most marked at 21 days, with an additional 11 patients experiencing symptom resolution for every 100 treated. In contrast, this effect was not significant at 15 days. Our subgroup analysis had only a small number of trials, however, and further research is needed to clarify the clinical benefit at 15 days or less (as discussed below). Clearly, patients are likely to experience pronounced symptoms in the first 7 to 14 days of their illness and may be less willing to consider a therapy that does not offer an increased likelihood of improvement in this earlier time period.
We found evidence of a dose-response relationship for mometasone furoate nasal spray: larger doses were associated with a greater likelihood of symptom resolution. We had insufficient data to assess whether other types of intranasal corticosteroids showed a similar effect, or whether this higher dose was associated with an increase in adverse events. On the basis of our review, when intranasal corticosteroids are used, we recommend doses of 800 μg of mometasone furoate daily.
Comparison With Existing Literature
The small benefit of intranasal corticosteroids for the broad measure of symptom resolution or improvement at 14 to 21 days was similar in direction and size to that found in a recent Cochrane review.13 In both cases, however, marked heterogeneity was present. We have demonstrated that this heterogeneity arises from both the variation in the timing of the outcome measure and the dose of intranasal corticosteroids used. We found larger effect sizes in subgroup analyses by dose and timing of outcome measure. The recent Cochrane review may therefore have underestimated the benefit of intranasal corticosteroids. Williamson et al14 acknowledged that their RCT was underpowered to detect clinically useful effects, and the study may have used an inappropriately low dose of budesonide.26
Limitations
Important limitations of this systematic review include first, that 5 of the studies prescribed antibiotics to both steroid and placebo groups. Williamson et al14 found no interaction between antibiotic therapy and steroid therapy using a factorial design, which argues against a synergistic effect of these drug classes.
Second, included studies varied in the types and doses of steroids, duration of therapy, and outcome measures reported. Particularly, the definition of resolution of symptoms varied among the studies, and all measures of resolution involved subjective assessment. These factors prevented pooling of all outcomes and are likely to have contributed to the heterogeneity of the data.
Third, included studies were underpowered to detect rare adverse effects of corticosteroid, as well as relapse rates and days missed from work or school. Fourth, the limited number of trials meant we were unable to assess publication bias using funnel plots or place undue weight on the findings from small subgroup analyses. Finally, in 4 of the 6 included trials, radiologic or endoscopic evidence of acute sinusitis was an inclusion criterion. In ambulatory care, it is impractical and inappropriate to perform radiologic investigations on patients with symptoms of sinusitis.
Recommendations for Research
This review highlights the need for adequately powered RCTs comparing intranasal corticosteroids with placebo in the absence of antibiotics for symptom relief in acute sinusitis. We recommend that trials should use at least 21 days of therapy with high-dose mometasone furoate nasal spray. Inclusion criteria should be based on a clinical scoring system rather than radiologic evidence. Self-report and telephone follow-up should be used to assess the time to complete resolution of symptoms and also the time to onset of symptom resolution, which will be particularly important in clarifying whether there is benefit at time points earlier than 21 days. Recording the duration of symptoms at baseline will also improve our understanding of patterns of symptom resolution.
As acute sinusitis is diagnosed in an estimated 31 million Americans annually,1 a full assessment of economic implications is important. Such assessment should look at the cost of 21 days of therapy with high-dose mometasone furoate (equivalent to 3 bottles containing 140 × 50-μg doses) and the indirect cost savings in terms of attendance and performance at work or school and quality of life measures (eg, with the SNOT-20 score).24 These data will improve our understanding of whether the small benefit of this therapy for the individual has larger benefits at the population level. Antibiotics are widely prescribed for acute sinusitis despite limited evidence of beneficial effect; thus, measuring the extent to which intranasal corticosteroids reduce antibiotic prescribing will be highly relevant to clinical practice and policy. A systematic review using individual patient data may improve our ability to combine the data from existing research. Finally, a double-blind, placebo-controlled trial of the benefit of oral steroids in acute sinusitis has not yet been performed. Since delivery of intranasal corticosteroids to the nasal mucosa may be reduced by nasal congestion, and this may be a factor responsible for our finding of a nonsignificant benefit at 15 days, oral drug delivery might offer earlier and greater symptomatic relief.
In summary, on the basis of the current evidence, we believe that intranasal corticosteroids offer a small therapeutic benefit in acute sinusitis and may be most helpful for symptoms of facial pain and nasal congestion. This benefit may be greater with courses of 21 days in duration and with high-dose mometasone furoate. Future trials in antibiotic-naïve patients that clarify the time-course of clinical benefit and the impact on work and quality of life will be important to guide management of this common condition in family practice.
Acknowledgments
We would like to acknowledge the input of Professors Paul Glasziou and Chris Del Mar into the initial stages of this project.
Footnotes
-
Conflicts of interest: authors report none.
-
Disclaimer: Neither the British Society for Antimicrobial Chemotherapy nor the National Institute of Health Research had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
-
Funding support: Funding for this work was provided in part by a grant from the British Society for Antimicrobial Chemotherapy Systematic Review Grant (GA722SRG). The Department of Primary Health care is part of the National Institute of Health Research (NIHR) School of Primary Care Research.
- Received for publication April 20, 2011.
- Revision received August 17, 2011.
- Accepted for publication September 6, 2011.
- © 2012 Annals of Family Medicine, Inc.