Abstract
PURPOSE To determine whether acute respiratory infection (ARI) decision aids and a general practitioner (GP) training package reduces antibiotic dispensing rate and improves GPs’ knowledge of antibiotic benefit-harm evidence.
METHODS A cluster randomized trial of 27 Australian general practices (13 intervention, 14 control) involving 122 GPs. Intervention group GPs were given brief decision aids for 3 ARIs (acute otitis media, acute sore throat, acute bronchitis) and video-delivered training. Primary outcome was dispensing rate of target antibiotic classes (routinely used for ARIs), extracted for 12 months before, and following, randomization. Secondary outcomes were GPs’ knowledge of antibiotic benefit-harm evidence; prescribing influences; acceptability, usefulness, and self-reported resource use; and dispensing rate of all antibiotics.
RESULTS The baseline mean dispensing rate of ARI-related antibiotics was 3.5% (intervention GPs) and 3.2% (control GPs) of consultations. After 12 months, mean rates decreased (to 2.9% intervention; 2.6% control): an 18% relative reduction from baseline but similar in both groups (rate ratio 1.01; 95% CI, 0.89-1.15). Greater increases in knowledge were seen in the intervention group than control; a significant increase (average 3.6; 95% CI, 2.4-4.7, P <.001) in the number of correct responses to the 22 knowledge questions. There were no between-group differences for other secondary outcomes. The intervention was well received, perceived as useful, and reported as used by about two-thirds of intervention GPs.
CONCLUSIONS A brief shared decision-making intervention provided to GPs did not reduce antibiotic dispensing more than usual care, although GPs’ knowledge of relevant benefit-harm evidence increased significantly.
- anti-bacterial agents
- decision making, shared
- decision support techniques
- general practice
- respiratory tract infections
INTRODUCTION
Antibiotic resistance is a rapidly growing international threat to health care. It is a direct result of antibiotic use and results in health resource waste and significant and avoidable health burdens.1 Reducing antibiotic use is central to minimizing the risk of resistance developing. Australia’s antibiotic use is high by international standards, with use in the top 25% of countries.2,3 Primary care, where antibiotics are used the most, is a priority target for reducing use. Acute respiratory infections (ARIs) are the most common indication for antibiotics, despite providing minimal benefits and some harms.3,4
Many patients believe antibiotics are necessary to resolve ARI symptoms,5,6 with patients overestimating the benefits and underestimating harms.5,7 Better management of patient expectations in ARI consultations may be important to reducing prescriptions as expectation exploration and management are often limited.8 General practitioners (GPs) are nearly 3 times more likely to prescribe antibiotics if they believe patients expect them,9 with perceived demand a significant and independent effect on prescribing.10 General practitioners’ expectations about antibiotic benefits and harms have not been studied, although they may similarly overestimate benefits and underestimate harms.11,12
Shared decision making provides a way to improve the accuracy of patients’ and clinicians’ expectations of benefits and harms. It enables GPs and patients to discuss the benefits and harms of using and not using antibiotics and to jointly decide on the most appropriate option.13 We have previously shown that three-quarters of people wanted more involvement in future decisions about the use of antibiotics for ARIs.5 Interventions which facilitate shared decision making for ARIs significantly reduced antibiotic prescribing in primary care.14 Most prior interventions, however, had multiple components and high intensity, limiting routine use. Patient decision aids are tools that facilitate shared decision making and are an effective way of integrating evidence with patient values and other factors into decisions.13 Decision aids, accompanied by brief instruction about use, may be a simpler, lower-cost way of promoting shared decision making about antibiotic use in a widespread and sustainable manner. In Australia, clinicians have limited opportunities for formal training in shared decision making.15
We systematically developed and piloted patient decision aids about antibiotic use for common ARIs. This randomized trial aimed to determine whether these aids and a brief training package for GPs reduced antibiotic dispensing rate and improved GPs’ knowledge of the benefits and harms of antibiotics for ARIs.
METHODS
The study was approved by the Bond University Human Research Ethics Committee (#15433).
Design
A cluster-randomized, pragmatic, parallel group trial, with 1:1 allocation of general practices to the intervention and control (usual care) groups, with 12-month follow-up.
Participants
Inclusion criteria were that at least 1 GP per practice consented to participation and that the practice was not currently and had not recently (within the last 2 years) been involved in any antibiotic use studies.
Recruitment
Practices were recruited using various methods, including approaching local practices and via a closed Facebook page of Australian GPs (Supplemental Appendix).
Randomization
The unit of randomization was the general practice; the unit of data collection was each GP’s consultation. Practices were randomized once all practice GPs had had the opportunity to consent. General practitioners needed to consent to trial participation and, on a government-approved form, to release their prescription data from the Pharmaceutical Benefits Scheme and number of consultations from the Medical Benefits Scheme for 24 months. Practices were randomized using a block-permuted design. The randomization sequence was produced using computer-generated random numbers by a trial statistician not involved in recruitment. The statistician provided allocation concealment and randomized the practices after receiving consent forms.
Intervention
The intervention consisted of previously piloted7 patient decision aids for 3 ARIs (acute otitis media, sore throat, acute bronchitis) and 15-minute video-delivered training for GPs (see Supplemental Table 1 for details of the intervention, its development, and piloting).
Control
The GPs were not provided the intervention package until after the follow-up interviews. In a decision beyond the study team’s control, the aids were released on a government department website during the trial, although the website is not targeted at GPs and the agency involved agreed to not directly promote the aids until the trial had concluded. We asked about potential contamination in follow-up interviews.
Outcome Measures
The primary outcome measure was the rate of antibiotic dispensing of the target antibiotic classes for each GP (expressed as consultations for which 1 of the target antibiotics was dispensed per 100 consultations). The target antibiotic classes were those routinely used for ARIs in Australia (cephalosporins, penicillins, macrolides). For each GP, we collected the number of target antibiotics dispensed (numerator of primary outcome measure) during the 12-months before and after randomization). These two 12-month periods were selected to account for any seasonality of antibiotic dispensing. For the same 2 periods, we also collected the total number of Medical Benefits Scheme-recorded consultations for each GP (denominator). By measuring dispensing rate, rather than prescribing rate, we were able to estimate actual antibiotic use more accurately as delayed prescribing is accounted for.
Secondary outcomes were GP knowledge of antibiotic use and benefit-harm evidence; prescribing influences; acceptability, usefulness, and self-reported resource use; and dispensing rate of all antibiotics. To assess GP knowledge about antibiotic use and antibiotic benefits and harms for ARIs, GPs completed a hard copy questionnaire after signing the trial consent form and at the 12-month follow-up interview. The completed questionnaires were returned to the research assistant in person or by e-mail. Questions were adapted from those used to pilot the decision aids7 and assess prescriber knowledge.11 The questionnaire consisted of 22 knowledge questions, 4 in yes/no format and 18 requiring a quantitative estimate. In addition, the questionnaire contained 9 questions regarding influences on prescribing in Likert-scale format where 1 indicated always and 5 indicated never. Data regarding acceptability, perceived usefulness, and self-reported use of resources for antibiotic prescribing in ARIs were collected in the follow-up interviews using open-ended questions. The rate of antibiotic dispensing of all antibiotics for each GP was also collected. General practitioners were asked to monitor for adverse events (see Supplemental Appendix for details).
Sample Size
We aimed to detect a relative rate reduction in dispensing of 20%, as a minimum clinically important difference. With 80% power, a significance level of 5%, and an intraclass correlation coefficient for the effect of clustering of 0.15, we calculated we would require 18 practices to detect this. (See Supplemental Appendix for further details.)
Analysis
Generalized estimating equations (GEE) negative binomial regression was used to compare mean dispensing rates between the intervention and control groups, adjusting for clustering by GP practice and baseline dispensing rate (data from 12 months before randomization). All practices were included on an intention-to-treat basis. Two sensitivity analyses were conducted, 1 for missing data for 1 GP, and 1 accounting for a possible 2-week delay in intervention commencement (see Supplemental Appendix, and Supplemental Table 2).
For the secondary outcome of GP knowledge, we compared the summed number of correct questions by group (scoring details in Supplemental Appendix), using GEE analysis of covariance to adjust for baseline and clustering by GP practice. Further, we compared the individual knowledge questions using clustered log binomial regression to estimate the relative proportion correct in the intervention and control groups (Supplemental Table 3). For Likert-response questions about antibiotic prescribing influences, we used GEE analysis of covariance to compare groups after adjusting for baseline values. Interview question responses were summarized, with descriptive statistics where possible, and open-ended question responses grouped according to response frequency.
RESULTS
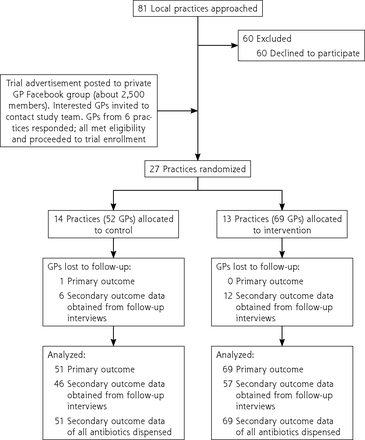
From May 2016 through May 2018, 27 practices (122 GPs) participated: 13 (69 GPs) in the intervention group and 14 (52 GPs) in the control group (Figure 1). The mean number of GPs per practice was 4.5 (range 1-14), 68 (56%) were female, and mean age was 47 (SD 10) years.
Trial flowchart.
Rate of Antibiotic Dispensing
At baseline, the mean dispensing rate of target ARI-related antibiotics was 3.5% of consultations (range 1.1% to 12.6%) for intervention GPs and 3.2% (range 0.7% to 9.7%) for control GPs. Over the follow-up period, mean rates decreased to 2.9% (range 0.7% to 11.5%) for intervention group GPs and to 2.6% (range 0.6% to 8.4%) for control group GPs (Table 1). These primary outcome results show an 18% reduction in dispensing rate from baseline to follow-up, but with no between-group difference. Sensitivity analyses are in the Supplemental Appendix.
Rate of Antibiotic Dispensing for Intervention and Control Groups
For the secondary outcome of rate of antibiotic dispensing of all antibiotics for each GP, there was a reduction in both groups, but no statistically significant between-group difference (Table 1).
Knowledge of Antibiotic Use and Benefits and Harms and Influences on Prescribing
At baseline, the mean (SD) knowledge score, out of a possible 22, was 7.4 (2.6) for the intervention group and 7.2 (2.3) for the control group. At follow-up, the intervention group’s mean was 11.0 (5.5) and the control groups was 7.3 (2.5).
After adjusting for baseline sum of correct responses and clustering within practices, there was a significant increase in correct responses in the intervention group of 3.6 more on average (95% CI, 2.4-4.7, P <.001) compared with controls.
When the quantitative estimate questions were considered individually, the intervention group had higher probability of correct responses, compared with the control group at follow-up, for 10 of the 18 questions (Figure 2). There was a wide range in the percentage of correct responses for these questions, with generally less than one-half of participants answering questions correctly (Supplemental Appendix). For the 4 general knowledge dichotomous questions, there was no significant between-group differences. For the questions about prescribing influences, there was no significant between-group difference (Supplemental Appendix).
Relative proportion, and 95% confidence interval, of correct response to individual knowledge questions, according to group allocation.
Acceptability, Perceived Usefulness, and Self-Reported Use of Resources
Of the 122 GPs, 103 completed the follow-up interview (57 intervention GPs, 46 control GPs), however not all answered every question. About two-thirds of intervention group GPs reported using the aids; the laminated document format was the most frequently used (35% of those who used them), 60% reported watching the training video, and 81% indicated they would continue using the aids after trial completion (Table 2). No control group GPs reported using any of the aids.
Intervention Group GPs Use of Decision Aids and Training Video (N = 57)
The 2 most frequent reasons given for not using the decision aids were satisfaction with current approach to antibiotic decision-making (“I am confident with my prescribing habits,” “my patients are happy with the decisions we make”) and perceived time constraints. The most common reason for not watching the training video was forgetting it was in the intervention pack.
Some GPs perceived the aids as helpful for structuring the discussion and for use with patients expecting antibiotics, although some felt using them was too time-consuming. General practitioners who used the aids reported most patients seemed to find them useful, appreciated the explanation, and some liked taking a copy. Some commented, however, that a few patients found parts of the information difficult to understand. Patient expectations for antibiotics was the most frequently mentioned factor influencing GPs’ decision to use an aid, followed by rapport with patients. General practitioners identified the 3 most useful aspects of the aids were as visual aids, providing conciseness and simplicity, and as a guide to structuring the consultation (Supplemental Table 4).
DISCUSSION
Summary of Main Findings
This cluster randomized trial that provided GPs with 3 patient decision aids intended for use in consultations with patients with ARIs found, at 12 months, a small reduction in antibiotic dispensing in both groups, but no between-group difference. For the secondary outcome of GP knowledge of antibiotic use and benefits and harms, however, there was a significant improvement in intervention group GPs. Generally, the intervention was well-received, perceived as useful, and reported to be used at least sometimes by about two-thirds of the intervention group GPs.
Strengths and Limitations
A strength is that there were almost no missing data for the primary outcome. Use of antibiotic dispensing (rather than prescribing) as a primary outcome measure is a strength as it accounts for delayed prescribing; important in Australia where patients can visit another GP for a prescription if not initially provided one. The use of a cluster trial, randomized by practice, enabled us to minimize contamination among GPs within a practice.
A limitation was our inability, due to budgetary constraints, to collect patient outcome measures for all patients who consulted participating GPs. Nevertheless, in conjunction with the trial, we conducted a nested observational study that involved some of the intervention and control group practices and a convenience sample of patients with an ARI. Full details are published elsewhere.16 In that study, 36 patients and 13 GPs consented to have the consultation audio recorded and analyzed by 2 independent assessors using the 12-item Observing Patient Involvement (OPTION-12) scale (measures the extent of shared decision making17) and 1 subscale of the Assessing Communication about Evidence and Patient Preferences (ACEPP) tool (assesses communication of the options’ benefits and harms).18 In 15/36 (42%) consultations in which an aid was used, mean observer-assessed shared decision-making scores were higher than in consultations that did not use an aid. Antibiotic harms were mentioned in all the consultations using an aid compared with mention in only 1of 21 usual care consultations.
We do not know which indication the antibiotics were prescribed for as we could not access GPs’ clinical records and these data are not captured in the Medical Benefits Scheme or Pharmaceutical Benefits Scheme databases. Access to records might have also enabled collection of data about adverse effects. Based on an earlier systematic review14 adverse effects were not expected, however, trial GPs did not return any adverse effect log forms. This trial was set against a dynamic background of various attempts to influence antibiotic use by clinicians and the public. Hence GPs in both groups may have been exposed to other sources of information regarding modifying antibiotic use during the trial. Dispensing rates, at baseline and follow-up, were substantially lower than expected based on data used for our sample size calculation (8% vs 3%) and there may have been little room for additional reduction in prescribing in this sample of GPs. As a small reduction in dispensing was seen in both groups and participating GPs had to provide consent for their prescribing data to be analyzed, their behavior may have been altered (Hawthorne effect).
Comparison With Other Literature
In contrast to the review of shared decision-making interventions for ARIs, which found a significant reduction in antibiotic prescribing in intervention groups,14 we found no significant effect. Most previous trials used more intensive and complex interventions. Seven of 9 studies included face-to-face interactive seminars and/or consultation skills training; about one-half provided skill feedback and/or a booster session; and the duration of training for most appeared to be at least 2-3 hours (range from 40-minute to >13 hours).14 A possible consequence of these time-demanding interventions is limited intervention uptake beyond the trials. To overcome such barriers, our intervention was very brief and GPs could view the 15-minute training package at their convenience. Only about 60% reported watching the video, however, and two-thirds used the aids. Our intervention may have been of insufficient intensity, had no planned reinforcement or reminders, and the training had no interactive components. Furthermore, our intervention did not focus on improving GPs’ attitude toward shared decision making nor provide the opportunity to practice skills and receive feedback. A positive attitude toward shared decision making, appreciation of how it differs from current practice, and developing skills in using decision aids may be just as important as providing the aids.19
Our results differ from those of a similar cluster randomized trial of an intervention (a diagnostic decision-support tool to help GPs estimate bacterial infection probability; a 2-hour online tutorial and 2-hour face-to-face interactive workshop).20 That trial found a significant difference in the patient-reported proportion of those who decided to use an antibiotic immediately post-consultation (27% in the intervention group vs 52% in the control group).20 Key differences are that we used an objective measure of antibiotic dispensing rather than patient self-report about immediate decision; collected data at the GP level; used a briefer training intervention; used decisions aids focused on antibiotic benefits and harms; did not address bacterial vs viral etiology; and our practices were not teaching practices with a single-university affiliation and high proportion of residents.
In the follow-up interviews, some comments from intervention group GPs reflected a paternalistic attitude to decision making that assumed an existing good relationship with patients obviated the need for decision-making support. Clinicians’ belief they already practice shared decision making is a common myth about and barrier to implementing it, along with the misperception that it takes too much time.13,21 Time constraint was the second most frequently reported reason for not using the aids.
None of the previous 105 randomized trials identified in the Cochrane review of decision aids reported measuring clinicians’ quantitative knowledge of the relevant benefit and harm evidence.22 Across nearly all questions in our trial, knowledge of antibiotic benefits and harms was incorrect for at least one-half of the GPs. We are not aware of other studies of clinicians’ quantitative knowledge of antibiotic benefits and harms, other than a survey of European primary care pediatricians which found almost 50% overestimated antibiotic benefits for preventing a complication of acute otitis media.11
Implications for Practice and Research
Despite not finding a significant effect on antibiotic dispensing, the patient decision aids should be made available for use in consultations as they were acceptable and useful according to most intervention group GPs and contributed to improved GP knowledge of the relevant evidence. Also, based on the nested observational study, they appeared to be associated with a greater extent of shared decision making in consultations.
Our novel finding of improved clinician knowledge suggests aids may have a previously unreported benefit for clinicians as they contain a concise summary of the benefit and harm evidence. Measuring clinician knowledge of benefits and harms as an outcome in future trials of patient decision aids would help to assess the replicability of this finding.
The training element of any clinician-facing intervention needs to strike a balance between being sufficiently intensive and interactive to achieve attitudinal and skill changes, but not too onerous that attendance or completion is inhibited. Future research could explore the minimum essential training components and format. For example, a 2-hour interactive e-learning course on shared decision making (which has a version for GPs) appears to improve clinicians’ shared decision-making confidence and knowledge, but behavioral effects are not yet tested.23
CONCLUSION
A brief shared decision-making intervention, consisting of ARI patient decision aids and a short (15-minute) video training session provided to GPs, did not reduce ARI-related antibiotic dispensing more than usual care, although GPs’ knowledge of relevant benefit-harm evidence increased significantly.
Acknowledgments
The authors thank Amanda Murray, Dr Laura Bergade, and Dr Elizabeth Gibson for their help as trial research assistants; Dr Malene Hansen for initial assistance with set-up of the trial recruitment systems and obtaining funding; and the administrators of GPs Down Under Facebook page for facilitating recruitment of GPs.
Footnotes
Conflicts of interest: T.C.H., C.DM., P.G., E.B., and L.T. declare support from the Natl Health & Medical Research Council of Australia (NHMRC) to conduct the trial; T.C.H. declares funding from the Australian Commission of Safety and Quality in Healthcare to update the patient decision aids after study completion. All authors declare no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Read or post commentaries in response to this article.
Funding support: This trial was funded by the Australian National Health and Medical Research Council (#1120433). The funder of the study had no role in considering the study design or in the collection, analysis, and interpretation of the data, writing of the report, or decision to submit the article for publication.
Trial registration: ACTRN12616000644460
- Received for publication January 20, 2021.
- Revision received May 14, 2021.
- Accepted for publication June 3, 2021.
- © 2022 Annals of Family Medicine, Inc.