Abstract
Promoting adherence to medical recommendations remains one of the oldest yet most persistent challenges of modern clinical practice. Although increasingly sympathetic to structural forces that affect health behavior, standard models frequently conceptualize nonadherence as a phenomenon of patient behavior, a self-evident quality belonging to patients that is responsible for a myriad of undesired outcomes. We contend, however, that this approach not only fails to consider the role of the clinician in the concept’s origins in clinical encounters, but also has facilitated the use of adherence terms (eg, nonadherent, noncompliant, treatment resistant) as pejorative social labels to the detriment of the physician-patient relationship. Used without care, such terminology can alter the meaning assigned to patients’ behaviors so that structural barriers to care such as poverty and systemic racism are reframed as problems of poor attitude or effort. This article explores the functions of adherence terms as social labels by reviewing their underlying logic in clinical settings and outlining pitfalls in the pathologization of nonadherence in research and practice. We propose the concept of adherence labeling—the assessment, classification, and dissemination of clinicians’ perceptions of patients’ adherence through social labels—as an alternative model to understand how adherence terms may inadvertently obstruct the care of marginalized patients.
- adherence
- patient noncompliance
- stigma
- bias
- bioethics
- systemic racism
- primary care issues: patient-centered care
- social labels
- physician-patient relations
- motivation
- barriers
- health behavior
- social marginalization
- minority groups
- vulnerable populations
INTRODUCTION
A Black woman aged 50 years with insulin-dependent diabetes experiences gaps in insurance related to unstable employment. During this period, she halves her insulin dose as a self-rationing strategy. Months later, she presents to her primary care clinic, where she is found to have progressive diabetic neuropathy and hyperglycemia. Her physician, influenced in part by implicit assumptions about race and individual responsibility, documents “poorly controlled diabetes due to nonadherence.” This description is viewed by other clinicians who draw similar conclusions about her willingness to participate in care. These feelings toward the patient affect her experiences in the clinic, leading her to feel alienated from both her physician and health care in general. What went wrong?
Encouraging adherence to treatment remains one of medicine’s oldest and most persistent challenges.1,2 Unfortunately, stigma has often accompanied descriptions of “nonadherent” patients with many scholars raising concern that such terms undermine patients’ autonomy and blame them for poor health outcomes.3-9 Although substantial effort has gone toward studying adherence as a behavior,10-12 much less has been written about adherence as a label—that is, how the term adherence and its derivatives assign meaning to patients’ behaviors. Several questions in this area remain unresolved. For example, how do such terms arise in clinical settings? How can nonadherence transform from something a patient does (nonadherent behavior) into something they are (a nonadherent patient)? Finally, what alternative (if any) could more appropriately capture this consequential clinical phenomenon?
Although labels may be a necessary part of clinical reasoning and practice, we argue that adherence terminology poses a unique risk to the care of socially vulnerable patients, for whom the stigma of terms such as nonadherent may outweigh their therapeutic promise. Here we lay conceptual groundwork necessary to understand how adherence labels can produce unintended harm among marginalized patients. To do this, we outline the logical underpinnings of adherence terminology and propose a new model for understanding the impact of these terms on clinical care that we call adherence labeling: the categorization of patients based on their perceived alignment with medical recommendations.
UNDERSTANDING ADHERENCE DOCUMENTATION
Although adherence has undergone extensive elaboration in today’s literature, most interpretations focus on patients’ behaviors; however, we believe that this approach does not adequately recognize the role of the clinician in the concept’s origins. In this section, we explore adherence as an idea emerging from the clinical encounter rather than as a self-evident fact pertaining to patients.
In an idealized setting, at least 3 conditions are necessary for adherence terminology to be used. First, there must be a recommendation that a clinician either provides or endorses. Second, a clinician must determine—by observation, self-reported or collateral history, prior documentation, or inference—whether a patient meets a particular standard with respect to that recommendation. Third, that clinician must decide whether their assessment of the patient’s adherence warrants explicit mention.
The process above illustrates several features not captured in standard adherence models. First, we see the meaning applied to patients’ attitudes and behaviors is of central importance—adherence both depends on and informs how clinicians perceive patients. Second, adherence terminology can be observed to operate along a categorical binary. Even if one chooses to describe patients as having varying degrees of adherence, its relevance as a clinical problem—epitomized by the term nonadherence itself—implies a threshold below which lesser degrees of adherence should be considered problematic. Lastly, we see determinations of adherence not only depend on clinicians’ assessments but, in fact, take place exclusively on the clinician’s side. That is, patients may disclose or directly exhibit behavior that objectively contrasts with a clinician’s recommendations, but it is ultimately the clinician who decides whether such behaviors warrant the explicit use of adherence terms. Taken together, the construction of adherence—or the emergence of concepts such as nonadherence or a nonadherent patient in a clinical encounter—resembles that of a diagnosis: these terms arise as a means to categorize patients based on the observations and judgments of a clinician.
NONADHERENCE AS A DIAGNOSIS
Although nonadherence is not routinely discussed as a diagnosis, its tendency to be treated as one can be widely observed. Scholars have made repeated attempts to establish diagnostic criteria for nonadherence.10,13 Epidemiologically, adherence is studied under the same risk model applied to standard medical conditions whereby nonadherence is conceived as a risk factor for various clinical outcomes.14 Conversely, scholars have sought to identify measurable risk factors for nonadherence,15 some even attempting to pinpoint a genetic basis.16-19
In clinical practice, nonadherence is frequently mentioned in case presentations and problem lists alongside other diagnoses.20-25 This practice is facilitated by the inclusion of noncompliance as a billable diagnosis in the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Clinical Modification (ICD-10-CM).26 Practice guidelines instruct clinicians to be vigilant in recognizing nonadherence.10 Explicit references to the role of clinicians “diagnosing” nonadherence are common.27-29
Short of being formally classified as a disease, nonadherence has been subject to nearly every major biomedical tool from risk models and outcomes measurements to genetic studies. This observation highlights another feature of adherence terminology: the concept of adherence relies on the pathologization of nonadherence. That is, the emergence of adherence as a desired clinical outcome has led to the framing of its opposite—nonadherence—as pathological.
There are at least 2 problems with pathologizing nonadherence. The first problem is that doing so carries a risky assumption: that it is normal for patients to adhere. Although there is no doubt nonadherence can cause harm, it is also known that health behaviors—outside of medical conditions that limit executive function (eg, psychiatric illness, dementia)—are frequently, if not exclusively, rational.5 Behaviors characterized as nonadherent may represent a wide range of contextually appropriate decisions drawing from health-belief models, cultural or religious views, and experiences with poverty and systemic racism.30 Systemic racism in particular may constrain patients’ self-management behaviors while also driving medical mistrust.31 Framing nonadherence as pathological thus creates a pathway for patients’ social, cultural, and structural contexts to be obscured or, at worst, reframed as problems of insufficient effort.
The second problem with pathologizing nonadherence lies in its definition. In psychiatric nosology, behavioral diagnoses require particularly rigorous definition to reduce the risk of stigmatizing normal behavior.32 Despite decades of attempts, scholars have struggled to generate a consistent, operational definition of nonadherence that establishes exactly when a patient’s level of adherence should be considered problematic. Often, cutoffs are proposed for objective markers such as the medication possession ratio33; however, such metrics simply reinforce the concept of adherence as a categorical binary. Even among measures developed to capture varying degrees of adherence,12 such tools’ clinical applicability still relies on the development of cutoffs to serve as clinical standards.34 Further, despite validation in the adherence literature, many such tools have rarely been adopted into clinical practice.12,35
As it stands, there are no widely accepted criteria for nonadherence. This raises an important question: how are clinicians deciding who should receive a diagnosis of nonadherence? As any clinician can attest, the decision is left to the individual. This situation constitutes what we are calling the subjective dependence of adherence terminology—that absent an objective standard, determinations of adherence may depend disproportionately on clinicians’ subjective assessments.
It has been shown in health equity literature that clinicians’ attitudes toward patients depend on factors such as personal views, demographics, and clinician-patient identity concordance.36-40 Several studies have demonstrated a similar effect in perceptions of adherence. Clinicians are often inaccurate in their judgments of patients’ adherence when compared with objective measures.41,42 One qualitative meta-analysis by Brundisini et al43 found marked variability between clinicians’ understanding of their patients’ adherence. Similarly, van Ryn and Burke,44 Bogart et al,45 Lutfey and Ketcham,46 and Huizinga et al47 found disparities in clinicians’ perceptions of adherence, with Black, obese, and poorer patients more often viewed as less adherent. Two additional studies revealed disproportionate application of the ICD-10 code for noncompliance among Black and poorer diabetic patients despite well-controlled glycemic markers.24,25 Although research in this area is lacking, these findings support the hypothesis that clinicians rely on subjective assessments to determine patients’ adherence, even when contradicted by objective data.24,25
Nonadherence may resemble a diagnosis in practice, but it demonstrates at least 2 qualities that distinguish it from a true medical condition. First, nonadherence, lacking a single unifying biopathological mechanism, represents primarily social rather than biologic information. Second, nonadherence is not routinely diagnosed by objective criteria and thus may be vulnerable to subjectivity and bias.
NONADHERENCE AS A LABEL
In sociology, a label is a term that identifies a person based on observable qualities such as racial phenotype, class status, or behavior. Social labeling describes how actions considered disruptive to society undergo a process of public identification resulting in often pejorative terms.48 Examples include terms related to mental illness, immigration status, or sexual practices.49-51 By definition, social labels arise from a society’s reaction to certain behaviors—this reaction both produces these social categories and assigns them meaning. Social labels thus do not indicate a behavior is inherently problematic or immoral but instead reflect a particular dominant social value.52,53
It is a well-established value that patients ought to follow physicians’ recommendations.54 Throughout history, patients who have not followed medical recommendations have been considered ignorant, dangerous, or even criminal.3,4 Today, health care professionals continue to express frustration regarding nonadherent patients.55 Although “noncompliant,” “nonadherent,” and similar concepts have proven useful in the study of patients’ health behaviors, our current models fail to capture the role of these terms as social labels. We therefore propose the idea of adherence labeling. We define adherence labeling as the assessment, classification, and dissemination of clinicians’ perceptions of patients’ adherence through the use of social constructs such as “compliance” or any of its contemporary substitutes (eg, adherence, concordance, engagement).
At its foundation, adherence labeling involves categorization. Through it, patients are sorted as adherent or nonadherent at a clinician’s discretion. For patients to be labeled adherent or nonadherent, a clinician must not only perceive a patient’s level of adherence but also decide to name and document it; thus, beyond categorization, adherence labeling is a process of knowledge production—it allows clinicians’ subjective assessments of patients to become codified as legitimate clinical data.
Use of the term nonadherence allows consolidation of a wide variety of behaviors under a single concept. If a patient struggles to take medication because of structural factors such as poverty or racism and is labeled nonadherent, there is no guarantee an explanation of the specific driver of that patient’s behavior will be made evident to subsequent clinicians. Instead, a patient may be incorrectly assumed to be defiant or careless rather than a victim of structural inequity. Adherence labeling therefore not only generates information about patients, but also has the potential to modify the meaning of patients’ behaviors.
We may further imagine that nonadherence can be assigned to certain patients because of implicit biases. Once such a label is given to a patient, the biased origins of the term are likely to become lost. Instead, the label of nonadherence may be interpreted as a clinical fact belonging to the patient rather than to the clinician. To this end, adherence labeling also functions as a process of concealment so that potential prejudice involved in decisions to label patients may be hidden beneath the label itself.
CASE DISCUSSION: THE “NONADHERENT” DIABETIC PATIENT
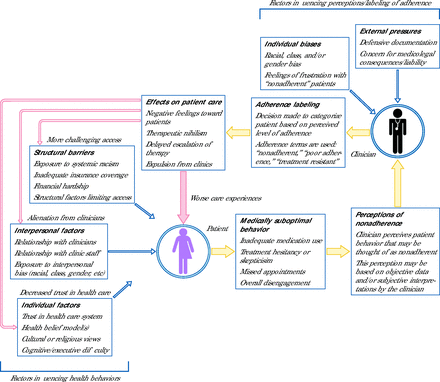
Returning to the case, we can observe that our patient was affected by racism through 2 interrelated mechanisms converging at her “nonadherence”: structural racism limiting access to therapy and interpersonal racism driving adherence labeling in her records. Not only does the patient’s unstable insurance lead to several negative health effects, but through adherence labeling, these outcomes are reimagined as evidence of her lack of engagement. Additionally, being labeled nonadherent led to worse care experiences that alienated the patient. This process itself may drive treatment hesitancy, which, in turn, may be seen as further evidence of her nonadherence. Our general concept of this cycle is shown in Figure 1. We use the term medically suboptimal to refer to behaviors that contrast with clinician recommendations but may draw from a competing logic or broader context.
Flowchart Illustrating Cyclic Effects of Adherence Labeling in Reframing and Potentially Exacerbating Existing Barriers to Medical Care
Note: Yellow arrows denote reframing influences; red arrows denote potentially exacerbating influences.
The meaning of this patient’s behavior underwent several transformations. Her rational decision to reduce her insulin was reframed as poor self-management. The negative outcomes she experienced were characterized as expected consequences of her carelessness. Lastly, the implicit bias that helped give rise to the label was obscured. These processes were contrived not only in the use of “nonadherence” but through the related phrase “poorly controlled diabetes.” We therefore note that adherence labeling may arise from any value statement applied to patients’ self-management. In summary, the patient has experienced multiple layers of harm, the meaning of which has been thoroughly modified by adherence terminology. Meanwhile, her social context—poverty, underinsurance, experiences of bias—has been hidden behind the sterile objectivity of the nonadherence label.
Notably, the case also highlights several areas of potential mitigation. Intentional inquiry could have elicited the rationale behind the patient’s behavior. Further, an awareness of stigma could have allowed the clinician to avoid unnecessary labeling and instead describe the patient’s behavior and its logic. Finally, these changes could have led to the design of a more patient-centered care plan focused on addressing the patient’s specific barriers to treatment.
CLINICAL IMPLICATIONS AND FUTURE DIRECTIONS
Few studies have investigated the clinical impact of adherence labeling. One reason may be that nonadherence is not commonly conceived as a label. Researchers may investigate behaviors labeled nonadherent, but rarely if ever examine how these labels come about or how they affect patient experiences and outcomes. Although limited, existing research on clinicians’ attitudes toward nonadherent patients provides some insight.
It is known that clinicians may view nonadherent patients negatively.8,56,57 Patients perceived as nonadherent may receive worse care including delays in necessary escalation of therapy for HIV and diabetes.58,59 Patients seen as uncooperative may be expelled from clinics or receive inadequate mental health care.60,61 Although system-level effects have not been explored, we might expect that labeling certain populations as nonadherent could impact decisions regarding resource allocation and program development. This influence would be particularly concerning considering the potential role of bias in individual adherence labeling. We suggest adherence labeling as a conceptual basis to investigate exactly which patients are being labeled nonadherent and how these terms affect both individual and macroscopic health care decisions. For example, vignette-based questionnaires could aim to measure the impact of adherence labels on clinical decisions. Additionally, studies could build on prior implicit bias literature to generate and test mitigation strategies for stigma associated with adherence terms.
Despite the potential harms of adherence labeling, we caution against the impulse to simply avoid adherence terms altogether. Rather, what is needed is a deeper understanding of the social and structural dynamics that underlie this labeling. For example, situating adherence labeling within incentive structures may help us understand the possible influence of outside pressures, such as concerns about liability that drive labeling as a form of defensive documentation. In addition, adherence labeling may be understood as drawing from the persistent cultural notion that patients should follow clinicians’ recommendations without question, an assumption that may explain why “nonadherence” can provoke frustration and judgment. Considering these factors is critical to envisioning an alternative approach to adherence that reduces stigma and emphasizes a more holistic and more forgiving view of patient behavior.
The goal of adherence documentation should be to accurately as well as equitably identify patients who need additional support to improve their disease management. Strategies for reducing the harms of adherence labeling should therefore focus on reimagining adherence documentation in such a way that emphasizes patient-specific barriers to care. Rather than characterizing patients as negligent, we might conceive of a version of adherence documentation in which these terms prompt clinicians to nonjudgmentally investigate the drivers of patients’ medically suboptimal behaviors. Alternatively, choosing language that simply states patients’ behaviors and their causes (eg, “low medication use due to underinsurance”) may help to reduce stigma and bring needed attention to patients’ modifiable social contexts.62 As highlighted in the presented case, care plans could be designed around patient-specific barriers to treatment through the use of validated interventions such as intensive outpatient case management, simplified regimens, or the addressing of medical racism.11,63,64 If clinicians can view terms such as nonadherence as opportunities to build a stronger therapeutic alliance with patients, especially with those already at the margins of our health care system, adherence terminology could possibly be leveraged to help close rather than exacerbate gaps in health equity. Clinicians should become more aware of the limitations and potential harms of adherence labeling in order to promote a more patient-centered, structurally informed approach to adherence.
Acknowledgments
S.B. would like to acknowledge Deren Pulley of the Department of Humanities and Social Sciences at the University of California San Francisco for his thoughtful support and encouragement.
Footnotes
Conflicts of interest: authors report none.
Author contributions: S.B. was primarily responsible for conceptualization and manuscript preparation. S.J.B. and P.F.C. oversaw the project and contributed to conceptualization and revision.
Previous presentations: Some of the content found in this article (including an earlier version of Figure 1) was presented in a poster session under the title “Who are we helping?—Ethical considerations in the documentation of patient refusal” at the Society of General Internal Medicine Annual Meeting; May 15-18, 2024; Boston, Massachusetts.
- Received for publication July 26, 2024.
- Revision received December 7, 2024.
- Accepted for publication January 20, 2025.
- © 2025 Annals of Family Medicine, Inc.