Abstract
PURPOSE Knee osteoarthritis is a common, debilitating chronic disease. Prolotherapy is an injection therapy for chronic musculoskeletal pain. We conducted a 3-arm, blinded (injector, assessor, injection group participants), randomized controlled trial to assess the efficacy of prolotherapy for knee osteoarthritis.
METHODS Ninety adults with at least 3 months of painful knee osteoarthritis were randomized to blinded injection (dextrose prolotherapy or saline) or at-home exercise. Extra- and intra-articular injections were done at 1, 5, and 9 weeks with as-needed additional treatments at weeks 13 and 17. Exercise participants received an exercise manual and in-person instruction. Outcome measures included a composite score on the Western Ontario McMaster University Osteoarthritis Index (WOMAC; 100 points); knee pain scale (KPS; individual knee), post-procedure opioid medication use, and participant satisfaction. Intention-to-treat analysis using analysis of variance was used.
RESULTS No baseline differences existed between groups. All groups reported improved composite WOMAC scores compared with baseline status (P <.01) at 52 weeks. Adjusted for sex, age, and body mass index, WOMAC scores for patients receiving dextrose prolotherapy improved more (P <.05) at 52 weeks than did scores for patients receiving saline and exercise (score change: 15.3 ± 3.5 vs 7.6 ± 3.4, and 8.2 ± 3.3 points, respectively) and exceeded the WOMAC-based minimal clinically important difference. Individual knee pain scores also improved more in the prolotherapy group (P = .05). Use of prescribed postprocedure opioid medication resulted in rapid diminution of injection-related pain. Satisfaction with prolotherapy was high. There were no adverse events.
CONCLUSIONS Prolotherapy resulted in clinically meaningful sustained improvement of pain, function, and stiffness scores for knee osteoarthritis compared with blinded saline injections and at-home exercises.
INTRODUCTION
Knee osteoarthritis is a chronic disease resulting in joint pain, stiffness, and decreased function.1 It is common, expensive for patients2 and society, and age-related3; by age 65 years, most of the population has radiographic evidence of osteoarthritis.4 Sources of pain include intra-articular and supportive extra-articular structures.5,6 Standard-of-care is multidisciplinary; however, a recent systematic review reported no clear benefit of any one therapy.4 Conservative therapies7 and oral supplements8,9 have been evaluated but are without clear efficacy. The Agency for Healthcare Research and Quality has called for the development of new therapies to prevent and treat knee osteoarthritis.4
Prolotherapy is an injection therapy for chronic musculoskeletal injury, including knee osteoarthritis.10–12 A core principle is the injection of small volumes of an irritant solution at multiple painful ligament and tendon insertions and in adjacent joint spaces over several treatment sessions.10 Prolotherapy has been used in a form recognizable to contemporary practitioners for at least 75 years; the earliest substantive report appeared in the allopathic literature when the technique was referred to as sclerotherapy as a result of the scar-forming properties of early injectants.13 Contemporary injection techniques were formalized in the 1950s, when the more commonly used term prolotherapy (from proliferant therapy) was adopted based on the observation that a larger cross-sectional area of ligamentous tissue was seen after prolotherapy injection in animal models.14 Literature of generally low methodological rigor from the 1930s to the early 2000s reported positive clinical outcomes.15 The mechanism of action is unclear. Contemporary hypotheses suggest that prolotherapy stimulates local healing of chronically injured extra- and intra-articular tissue, though definitive evidence is lacking.10 Hypertonic dextrose is a commonly used injectant.10 Prolotherapy injections target multiple potential pain generators in and around the knee joint; it may be well-suited to address the multifactorial cause of knee pain from osteoarthritis. A single randomized controlled trial (RCT)11 and 1 open-label study16 reported improvement in outcomes in response to prolotherapy but were not methodologically rigorous. We therefore conducted a 3-arm RCT to assess the hypothesis that adults with symptomatic knee pain receiving prolotherapy will report greater improvement in knee-related quality-of-life than those receiving saline injections or at-home knee exercises.
METHODS
The study was approved by the University of Wisconsin (UW) Health Sciences Institutional Review Board. Adults aged 40 to 76 years were recruited from 2004 to 2009 from the community and University of Wisconsin family medicine, sports medicine, and rehabilitation clinics; each was then observed for 1 year. Inclusion criteria were a diagnosis of knee osteoarthritis based on clinical criteria (American College of Rheumatology),17 identification of knee osteoarthritis by a radiologist on an existing knee radiograph obtained within 5 years of enrollment, tenderness of 1 or more anterior knee structures on physical examination, and self-reported moderate-to-severe knee pain for at least 3 months, defined as a score of 3 or more (0 to 6 ordinal response scale) on the question, “What is the average level of your left/right knee pain over the last week?” Exclusion criteria included pregnancy, diabetes, anticoagulation therapy, history of total knee replacement, prior knee prolotherapy, any knee injection within 3 months, inflammatory or postinfectious knee arthritis, daily use of opioid medication, allergy or intolerance to study medication, body mass index (BMI) greater than 40 kg/m2, and comorbidity severe enough to prevent participation in the study protocol, including at-home exercise or attendance at scheduled injection appointments. Each knee was assessed separately for eligibility. Interested, eligible persons attended an informational meeting, gave consent for participation, and were enrolled.
Study Design
Participants were randomly assigned to 1 of 2 injection groups (dextrose or saline) or exercise using a computer-generated randomization scheme in forced blocks of 6 prepared by the UW Pharmacy Research Center. The injector, outcome assessor, principal investigator, and participants were blinded to injection group status.
Injection Intervention
Injections were performed at 1, 5, and 9 weeks with optional additional sessions at 13 and 17 weeks per the physician’s (J.J.P.) recommendations and the participant’s preference. Before the procedures the off-site UW Pharmacy Research Center prepared dextrose and saline syringes that were blinded using an opaque paper sleeve. Participants were offered an optional single 5-mg oxycodone tablet 30 minutes before injection. The injector (J.J.P.) examined the knee, marked tender anterior knee locations, placed anesthetic skin wheals of 1% lidocaine, and performed extra- and intra-articular injections according to a published protocol (Table 1).16 Extra-articular injections were done on bone by palpation at major tender tendon and ligament insertions through up to 15 skin punctures using a peppering technique, placing a possible total 22.5 mL of solution; ultrasound guidance was not used. The 6-mL intra-articular injection was then delivered using an inferomedial approach. After the injection, participants were offered acetaminophen and 8, 5-mg oxycodone tablets to use as needed for up to 1 week and were advised on relative knee rest for 2 to 3 days with progressive resumption of routine activity over 1 month. They were discouraged from using nonsteroidal anti-inflammatory medications (NSAIDs) and from starting new therapies for their osteoarthritis during the study period.
Injection Solutions and Injection Techniques
At-home Exercise Intervention
Exercise group participants received an informational pamphlet about knee osteoarthritis (Visual Health Information, at http://www.vhikits.com/Default.aspx) depicting 10 at-home knee exercises demonstrated by the study coordinator at baseline. Participants were advised to begin exercises (3 sessions per week, 1 session daily, 10 repetitions per exercise), to gradually increase therapy as tolerated over 20 weeks (5 sessions per week, 3 times daily, 15 repetitions per exercise), and to continue them thereafter if desired.
Adherence and Precautions
Exercise group adherence was encouraged and assessed during telephone call reminders at the same interval that injection sessions occurred. Members of all groups were cautioned at each contact against knee overuse.
Outcome Measures
The primary outcome measure was change in knee-related quality-of-life as assessed by the composite score of Western Ontario McMaster University Osteoarthritis Index (WOMAC), a validated questionnaire evaluating osteoarthritis severity using pain, stiffness, and function subscales.18 The WOMAC composite score, constructed as the weighted average of the 3 subscale scores, ranges from 0 (worst) to 100 (best) knee-related quality-of-life19 and has been shown to be responsive to change.18 The minimal clinical important difference (MCID) on the WOMAC for knee osteoarthritis has been reported as 12 points of change on a 0- to 100-mm visual analog scale.20,21 Secondary outcomes included the knee pain scale (KPS),22 a validated questionnaire assessing knee pain frequency (0 to 4 ordinal scale) and severity (0 to 5 ordinal scale), with higher values indicating worse symptoms. KPS data were collected separately for each treated knee and for untreated knees. The WOMAC and KPS scores were collected in person and before any procedure at baseline, 5, 9, and 12 weeks, and by telephone at 26 and 52 weeks.
Tertiary outcomes for injection participants included (1) ratings of procedure-related pain severity, using a 1 to 7 ordinal scale, obtained immediately after and 2 days after each injection session; and (2) daily logs of opioid medication use (yes/no) during the 7 days after each injection. Treatment satisfaction was assessed among all participants at 52 weeks with the question, “Would you recommend the therapy you received in this study to others with knee osteoarthritis like yours? (yes/no).” All participants were able to make brief qualitative comments about their experiences.
Demographics, self-reported weight and height, and severity of knee osteoarthritis seen on knee radiographs were collected at baseline to characterize the sample and to evaluate as covariates for statistical analysis. A fellowship-trained musculoskeletal radiologist (R.K.), using the 1- to 4-point Kellgren-Lawrence knee osteoarthritis scoring system,23 evaluated existing, available knee radiographs. Attendance at injection sessions was tracked. Adherence to at-home exercises was assessed by the question, “In the past month, did you perform home exercises as directed? (yes/no),” administered by monthly mail-in logs for the first 20 weeks of the study. Blinding of the injector and injection participants was assessed at each injection session by asking each to identify the participant’s group assignment using the items “dextrose,” “saline,” or “don’t know.”
Analysis
Two RCTs and clinical experience guided a priori sample size calculations. One RCT assessing prolotherapy for knee osteoarthritis reported a 44% effect size compared with baseline status on a visual analog scale.11 A well-designed RCT reported a 20% to 40% effect size of prolotherapy for low back pain.24 Assuming minimal change in the control groups and minimal loss to follow-up, 32 participants per arm would provide 80% power to detect a 20% difference in mean composite WOMAC scores between control and dextrose participants at a significance level of 5%.
Data were analyzed using SAS 9.1 statistical software (SAS Institute Inc). Descriptive statistics describe outcomes at each time point; mean value and standard deviation (SD) were reported at baseline.
Analysis was by intention-to-treat. Repeated measures analysis of variance compared treatment groups on follow-up WOMAC total and subscale scores and KPS subscales after adjusting for baseline scores, age, sex, and BMI. Statistical significance between treatment groups was assessed at each time point (group × time interaction) and comprehensively for the entire time frame (main time effect). Because the WOMAC evaluates participant’s knee-specific quality-of-life not considering the number of knees affected, the unit of analysis of the WOMAC scores was the participant regardless of the number of knees injected. Percentage improvement in WOMAC scores was calculated as the percentage change in total WOMAC score from baseline to 52 weeks relative to baseline score.25,26 The proportion of participants in each group who met the MCID benchmark of 12 points on the 0- to 100-point composite WOMAC was calculated.
The unit of analysis for the KPS model was the individual knee. Because each participant completed 2 KPS questionnaires at each time point—1 per knee, the KPS scores for each knee were analyzed individually. If a participant had both knees treated, that participant accounted for 2 knees in the treated knees model. A hierarchical repeated measures model corrected the standard errors for the interaction between the reports on 2 knees by the same individual. A separate repeated measures model analyzed KPS scores for single untreated knees. The significance test for change from baseline is reported for WOMAC and KPS scores. A 2-tailed P value <.05 was established as a statistical significance level.
RESULTS
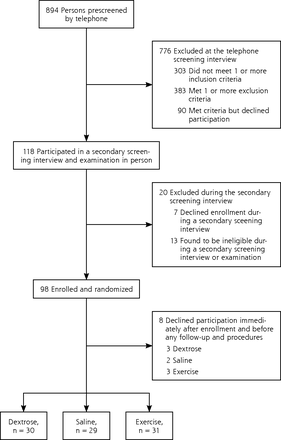
Of the 894 persons screened by telephone, 118 met initial eligibility criteria; 98 persons were enrolled and randomized. Eight enrollees dropped out before completion of any procedures or follow-up data collection. Ninety participants were therefore included in the analysis (Figure 1).
Screening, enrollment, and randomization.
There were no significant baseline differences between groups (Table 2). The 8 enrollees who withdrew before the follow-up procedures were women; there were no other differences between the 8 women and the analyzed sample. The study sample consisted of 66% women with a mean age of 56.7 years (SD = 7.2 years); 74% were either overweight (BMI ≥25–29.9 kg/m2) or obese (BMI ≥30 kg/m2). Participants reported more than 5 years of knee pain, and most had failed at least 1 conservative therapy. Although radiograph reports identifying osteoarthritis were available for all included knees, administrative difficulties resulted in procurement of only 68 prestudy radiographs. The Kellgren-Lawrence scores ranged from mild to severe, and overall inclusion criteria, x-ray reports, and baseline WOMAC scores19 suggest that on average, this cohort had moderate severity of knee osteoarthritis (Table 3).
Baseline Participant Characteristics by Treatment Group
Baseline Participant Knee Osteoarthritis Severity Scores by Treatment Group
Dextrose participants received 3.95 ± 1.0 injection sessions; 13 participants had both knees treated (26 knees), and 17 participants had 1 knee treated (total 43 knees). Saline participants received 3.71 ± 1.1 injection sessions; 13 participants had both knees treated and 15 participants had 1 knee treated (total 41 knees). Exercise participants returned an average of 22 (77.4%), self-assessments during the 20-week treatment period; 77% of participants reporting doing their at-home exercises as directed; 16 participants had both knees treated and 15 participants had 1 knee treated (total 47 knees). Fourteen participants reported using NSAIDS in the dextrose and saline groups, whereas 15 exercise participants reported NSAID use.
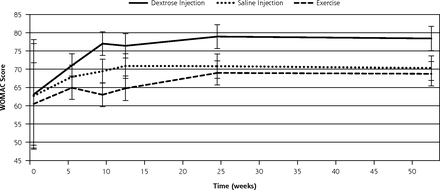
Between-group comparisons showed that dextrose participants at 52 weeks reported improved composite WOMAC scores (15.32 points, a 24% improvement compared with baseline status) compared with participants in the saline (7.59 points; P=.022) and exercise groups (8.24 points; P = .034). Fifty percent (15 of 30) of the dextrose participants improved by 12 or more points on the composite WOMAC score at 52 weeks compared with 30% (10 of 29) of saline participants and 24% (8 of 31) of exercise participants. Significant differences were also found at 9 weeks for dextrose compared with saline and exercise groups, 13.91 points compared with 6.75 points (P=.020) and 2.51 points (P=.001), respectively; and at 24 weeks, changes of 15.85 points compared with 8.12 points (P = .021) and 8.48 points (P=.024), respectively (Table 4, Figure 2).
Change in the WOMAC Composite and Subscale Scores Over Time
Change in WOMAC composite scores over 52 weeks (± standard error).
WOMAC = Western Ontario McMaster University Osteoarthritis Index.
Note: WOMAC is scored on a range of 0 to 100 points, with higher scores indicating better knee-related quality of life. Nonoverlapping confidence intervals indicate significance of change in dextrose scores compared with change in scores of both saline (P <.05) and exercise (P <.05) groups.
Evaluation of the WOMAC subscale scores showed that dextrose participants generally reported consistent improvement across the subscales, achieved near-maximum improvement by 26 weeks, and remained stable through 52 weeks. The most dramatic improvements were on the function subscale; dextrose participants reported significantly better function than both saline and exercise participants for a change of 16.25 compared with 5.46 (P = <.001) and 7.31 points (P = .009), respectively, at 52 weeks.
At 9 weeks, dextrose participants reported significantly better function than both saline and exercise, with a change of 13.58 compared with 5.85 points (P = .021) and 4.00 points (P = .004), respectively.
At 24 weeks, dextrose participants also reported significantly better functional change than both saline and exercise, with a change of 17.19 points compared with 7.62 points (P = .005) and 9.30 points (P = .018), respectively.
There was no correlation between exercise compliance in the exercise group and WOMAC composite improvements at 52 weeks (r = −0.11, P = .625).
Overall, the WOMAC scores of saline participants did not significantly differ from those of the exercise group except for the stiffness scores at 9 (P=.047) and 12 weeks (P = .049), when the saline group fared better. Regardless of the number of knees injected, KPS-based knee pain frequency (9 through 52 weeks, P <.05) and severity (24 and 52 weeks, P <.05) were significantly reduced in the dextrose group compared with both comparison groups (Table 5). KPS scores of untreated knees improved slightly in all 3 groups compared with baseline but were not different between groups.
Change in Knee Pain Scale Pain Frequency and Pain Severity Scores in Individual Treated Knees Over Time
All injection group participants experienced expected mild to moderate postinjection pain; 3 participants in the dextrose group and 5 in the saline group experienced self-limited bruising. There were no other side effects or adverse events. The use of periprocedural analgesics was not different between injection groups. Sixty-three percent of saline participants used acetaminophen before or after injection compared with 74% of dextrose participants. Oxycodone was used before (63%) and after (47%) dextrose sessions and before (57%) and after (43%) saline injection sessions. Ninety-one percent of dextrose participants, 82% of saline participants, and 89% of home exercise participants reported they would recommend their respective interventions to other patients with knee osteoarthritis. Blinding was intact; the injector indicated “don’t know” 93% of the time, and participants indicated “don’t know” 91% (dextrose) and 93% (saline) of the time, with the remaining selections evenly divided between correct and incorrect answers (P = .77).
DISCUSSION
This RCT of adults with symptomatic knee osteoarthritis found substantial, consistent, and significant improvements in composite WOMAC scores at 26 and 52 weeks for the dextrose group compared with saline injections and at-home exercise groups. At 52 weeks, the average improvement on the WOMAC score was 15.32 ± 3.3 points or 24% compared with the baseline score; 50% (15 of 30) of the dextrose group reported improvement in the composite WOMAC score for the dextrose-treated participants, which exceeded the MCID of 12 points. Improvement in the dextrose group was consistent across the 3 WOMAC subscales, was nearly maximum by 26 weeks, and remained stable through 52 weeks. KPS-based results on a per knee basis were consistent with WOMAC findings.
These effects are consistent with findings of a single-arm prospective study (N = 36) using an identical injection protocol and similar eligibility criteria.16 Participants in that study were slightly more symptomatic at baseline but reported similar overall effects at 52 weeks on WOMAC and KPS outcome measures; uninjected contralateral knees also showed significant improvement, suggesting that dextrose prolotherapy for more symptomatic knee osteoarthritis may also result in improvement of the uninjected side, likely through reduction in compensatory mechanisms. Our current findings are also consistent with a second prolotherapy RCT for knee osteoarthritis, though comparison is limited by methodological heterogeneity.11 Direct comparison with studies of hyaluronic acid injection or other therapies is also limited given the heterogeneity of study eligibility criteria, overall health status, patient expectation, baseline osteoarthritis severity,21 and WOMAC scoring methodology,27 but improvements of 20% to 40% compared with baseline have been reported.4,28
The mechanism of action for dextrose is unclear. Hypertonic dextrose has been hypothesized to stimulate healing of chronically injured extra- and intra-articular tissue29; animal model studies reported increased inflammatory markers30 and significantly enlarged cross-sectional area in medial collateral ligaments.31 The potential of prolotherapy to stimulate release of growth factors favoring soft tissue healing11,32 and a positive neural effect33 have also been suggested. In addition to dextrose-specific effects, needle trauma and volume expansion of local tissue may also produce tissue-level effects.34
Limitations of this study include a relatively small sample size, though the effect size of prolotherapy proved adequate to detect between-group differences. The study was not large enough to detect uncommon adverse events, such as intolerance to study medication or rare injection-related sequelae. Generalizability may be limited by numerous exclusion criteria, the relative youth of the cohort compared with those in some knee osteoarthritis studies,35 and the relative lack of participants with very severe baseline WOMAC scores. The assessment of participant satisfaction was indirect and subject to bias. Radiographs were not available for all participants, and the use of Kellgren-Lawrence criteria for baseline radiological assessment of knee osteoarthritis severity is controversial. The Kellgren-Lawrence score, however, is likely to remain an important measure for gauging disease severity in symptomatic patients.36 The exclusion of patients taking chronic opioids limits the generalizability of the results. Although clinical experience suggests that such patients may still benefit from prolotherapy, long-term (greater than 1 year) effectiveness and side effects are unknown. Strengths include pragmatic assessment using validated, patient-oriented outcomes and robust, consistent results.
These findings suggest that dextrose prolotherapy may improve upon standard care of knee osteoarthritis for certain patients. Its use in clinical practice is relatively uncomplicated; prolotherapy is performed in the outpatient setting without ultrasound guidance using inexpensive solutions. The knee protocol is easy to learn and requires less than 15 minutes to perform; continuing medical education is provided in major university and national physician organizations settings.10 A prior study suggested that clinical improvement may accrue preferentially to those who are middle-aged, of normal BMI, and female.16 For responders, whether prolotherapy results in sustained effect past 52 weeks, disease modification, or delayed definitive care, such as knee replacement, is not known. Clinical experience suggests that repeated sessions and tune-up sessions after 52 weeks improve outcomes and do not pose additional risk. The described procedure costs $218 per session in our clinic. Some third-party payers cover prolotherapy with authorization, but most patients pay out-of-pocket. Interest in prolotherapy among physicians and patients in the United States appears to be high based on attendance at continuing medical education conferences and physician listings on relevant websites.10 Although the number of practitioners who perform prolotherapy in the United States is likely in the hundreds, no formal survey has been done since 1993.37
Prolotherapy for knee osteoarthritis has not been compared with other current therapy, including intra-articular corticosteroid and hyaluronic acid injections. Determination of clinical utility of prolotherapy will require confirmation in a larger effectiveness trial that includes biomechanical and imaging outcome measures to assess potential disease modification.38,39 Clinical trials designed to optimize dose and assess biological mechanism of action are also warranted.
Prolotherapy performed by a trained operator resulted in safe, significant, and sustained improvements on validated, quality-of-life, pain, function, and stiffness measures compared with blinded (saline injections) and nonblinded (at-home exercise) comparison interventions. Prolotherapy may be an appropriate therapy for patients with knee osteoarthritis refractory to conservative care.
Footnotes
-
Conflict of interest: authors report none.
-
Previous presentations: Parts of the current paper have been presented in 2 peer-reviewed conference settings (poster and podium) as below: Rabago D, Miller D, Zgierska A, Mundt M, Kijowski R, Belling J, Patterson, JJ; Dextrose prolotherapy for knee osteoarthritis: Results of a randomized controlled trial (Poster presentation); Osteoarthritis Research Society International (OARSI) World Congress on Osteoarthritis; San Diego California, September 15-17, 2011.
-
Rabago D, Zgierska A, Mundt M, Kijowski R, Belling J, Patterson, JJ; Dextrose prolotherapy for knee osteoarthritis: Results of a randomized controlled trial (Oral presentation); North American Primary Care Research Group (NAPCRG) 39th annual conference; Banff, Canada; November 12-14, 2011.
-
Clinical trials identifier: NCT00085722
-
Funding support: National Institutes of Health: National Center for Complementary and Alternative Medicine: 5K23AT001879-02. The funding source had no role in study design, collection and analysis of data, or in the writing of this report.
- Received for publication March 21, 2012.
- Revision received September 28, 2012.
- Accepted for publication October 19, 2012.
- © 2013 Annals of Family Medicine, Inc.