Abstract
PURPOSE Recent results suggests the risk of a new onset of depression increases with longer duration of opioid analgesic use. It is unclear whether new-onset depression related to opioid analgesic use is a function of the dose prescribed or the duration of use or both.
METHODS Using a retrospective cohort design, we collected patient data from 2000 to 2012 from the Veterans Health Administration (VHA), and from 2003 to 2012 from both Baylor Scott & White Health (BSWH) and the Henry Ford Health System (HFHS). Patients (70,997 VHA patients, 13,777 BSWH patients, and 22,981 HFHS patients) were new opioid users, aged 18 to 80 years, without a diagnosis of depression at baseline. Opioid analgesic use duration was defined as 1 to 30, 31 to 90, and more than 90 days, and morphine equivalent dose (MED) was defined as 1 to 50 mg/d, 51 to 100 mg/d, and greater than 100 mg/d of analgesic. Pain and other potential confounders were controlled for by inverse probability of treatment–weighted propensity scores.
RESULTS New-onset depression after opioid analgesic use occurred in 12% of the VHA sample, 9% of the BSWH sample, and 11% of the HFHS sample. Compared with 1- to 30-day users, new-onset depression increased in those with longer opioid analgesic use. Risk of new-onset depression with 31 to 90 days of opioid analgesic use ranged from hazard ratio [HR] = 1.18 (95% CI, 1.10–1.25) in VHA to HR = 1.33 (95% CI, 1.16–1.52) in HFHS; in opioid analgesic use of more than 90 days, it ranged from HR = 1.35 (95% CI, 1.26–1.44) in VHA to HR = 2.05 (95% CI, 1.75–2.40) in HFHS. Dose was not significantly associated with a new onset of depression.
CONCLUSIONS Opioid-related new onset of depression is associated with longer duration of use but not dose. Patients and practitioners should be aware that opioid analgesic use of longer than 30 days imposes risk of new-onset depression. Opioid analgesic use, not just pain, should be considered a potential source when patients report depressed mood.
INTRODUCTION
Depression co-occurs with chronic noncancer pain1,2 and is known to be associated with opioid use. Patients with chronic noncancer pain and depression are more likely than those without depression to receive opioids,3 have a longer duration of use,4,5 take them at higher morphine equivalent doses (MEDs),6 and misuse and or abuse opioids.7,8 A review of psychopathology in pain9 suggests the opioid epidemic in the United States reflects underdetected and undertreated mental illness in patients with chronic pain.
Every year more than 200 million prescriptions for opioids are written in the United States.10 Known consequences of this opioid epidemic include abuse and accidental overdose.11–13 A less understood adverse outcome and emerging line of inquiry is the association between opioid use and risk of depression. In a study of nearly 50,000 Veterans Health Administration (VHA) patients, opioid analgesic use of longer than 180 days, compared with 1 to 90 days, was associated with significantly greater risk (hazard ratio [HR] = 1.51; 95% CI, 1.31–1.74) of a new diagnosis for depression.14 In a prospective study of chronic pain patients, those who increased their MED to more than 50 mg/d, compared with nonusers, had a 2.6 times greater probability of depression with time.15 These results remained after rigorous control for confounding.
Depression may be associated with opioid resistance16 and with opioid misuse.17–19 The large population of patients receiving opioid therapy may experience a parallel, yet unrecognized, increase in depression. Firmly establishing that greater duration, dose, or both of opioid analgesic use are associated with a new onset of depression, and in diverse patient populations, can inform pain management and public health incentives. A fuller understanding of opioid-related depression is relevant to primary care in light of the increased provision of pain management in this setting.20
Our prior studies of the link between opioid analgesic use and depression have several limitations,14,15 including unknown generalizability to other US health care populations, and uncertainty as to whether duration and dose are both associated with depression. In addition, our previous study of VHA patients relied on diagnosis of painful conditions without patient-reported pain scores and was conducted using a cohort designed for studies of incident heart disease.21
In this study, we expand our previous research14,15 to determine (1) whether longer duration of opioid analgesic use is associated with new-onset depression while controlling for dose; (2) whether a higher dose is associated with new-onset depression after adjusting for duration; (3) whether opioid analgesic use remains associated with new-onset depression after controlling for pain scores in VHA patient data; and (4) and whether results generalize to 2 independent health care populations.
METHODS
Electronic medical record data were obtained from 3 separate health care systems: Veterans Health Administration (VHA), Baylor Scott & White Health (BSWH), and the Henry Ford Health System (HFHS). The latter 2 systems used data from the HMO Research Network Virtual Data Warehouse.22 Data included International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes; prescription records, laboratory results, and demographic information.
Cohort Eligibility
Samples were restricted to those aged 18 to 80 years at baseline and excluded those with a diagnosis of cancer or human immunodeficiency virus infection. Patients must have had at least 1 visit in each of the 2 washout years before the start of follow-up period during which cases of depression and opioid use were removed. The 2-year washout has been applied previously as a conservative time frame to remove patients with common conditions.21,23 Patients must have had at least 1 visit after the follow-up period started. Patients who initiated opioid analgesic use after the onset of depression were excluded because they did not provide information regarding a new onset of depression. The end of the follow-up period was a new onset of depression or last known visit in the follow-up period. These criteria set up the temporal relationship so that incident opioid use occurred in patients without current depression and preceded any new-onset depression. The follow-up period was 2002 to 2012 in the VHA, and 2005 to 2012 in BSWH and HFHS. Measures
Duration and Dose of Incident Period of Opioid Analgesic Use
Opioids included codeine, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, oxycodone, oxymorphone, morphine, and pentazocine. Patients were considered continuous users if no gap of more than 30 days occurred between the end of one prescription and the start of another. Duration was computed by summing the days’ supply variable that measures the days required to exhaust the medication if taken at the maximum dose prescribed. Patients were classified into 3 mutually exclusive groups: 1 to 30 days, 31 to 90 days, and longer than 90 days’ continuous supply. Opioid use of longer than 90 days was chosen as the upper threshold because too few patients were available in private sector samples to model subgroups beyond 180 days. Duration of use was computed until end of follow-up or the start of a longer than 30-day gap.
A morphine equivalent dose was calculated using standard equianalgesic conversion tables that provide the amount of morphine equivalent to the opioid in a given medication (eg, oxycodone). Daily dose was computed from days’ supply and total dispensed, assuming patients took the maximum dose prescribed per day. The MED of opioid on the last day before the end of the follow-up period or the last day before a longer than 30-day gap was used to assign patients to 1 of 3 mutually exclusive groups: 1 to 50 mg/d, 51 to 100 mg/d, and greater than 100 mg/d MED.
Outcome
New-onset depression was defined by 2 or more outpatient diagnoses (ICD-9-CM codes: 296.2, 296.3 and 311) within the same 12-month period or 1 or more inpatient diagnoses for depression. This diagnostic algorithm has a 99% positive predictive value for a chart-reviewed diagnosis,24 and an 88% positive predictive value and a 71% negative predictive value compared with self-reported lifetime of depression.25
Covariates
Except for pain scores and marital status, available only in VHA data, variables were available and measured the same way in each patient cohort. Demographics included age, sex, race, marital status, and insurance. Insurance was defined as federal coverage only (VHA and Medicare) vs other sources. ICD-9-CM codes were used to define posttraumatic stress disorder, any other anxiety disorder (a composite of panic disorder, generalized anxiety disorder, social phobia, obsessive compulsive disorder, or anxiety disorder not otherwise specified), alcohol abuse/dependence and illicit drug abuse/dependence (which includes opioid abuse/dependence), and nicotine dependence or history of smoking. Comorbid health conditions included type 2 diabetes mellitus, hypertension, cerebrovascular disease, low testosterone levels, sleep apnea, obesity defined by body mass index or ICD-9-CM code, and cardiovascular disease (a composite of hyperlipidemia, ischemic heart disease, disease of pulmonary circulation, other heart disease, hypertensive heart disease, or myocardial infarction).
Painful conditions comprised 5 categories: arthritis, back pain, headaches, musculoskeletal pain, and neuropathic pain, using previously reported ICD-9-CM codes for more than 900 conditions for which an opioid may be prescribed.14,26 In VHA data we adjusted analyses for maximum pain scores reported at any time before the end of the follow-up period. The pain score is collected by a stand-alone clinical instrument used in primary care visits. The score is on a 10-point scale, with higher scores indicating worse pain.
To adjust for detection bias, we created a health care utilization variable defined by quartiles of the average number of clinic visits per month.
Propensity Scores and Data Weighting
Bias by indication may confound the association between duration or dose of opioid analgesic use and new-onset depression. To observe the direct contribution of opioid use to new-onset depression, potential confounders were balanced across levels of duration of opioid use and levels of MED using propensity scores. The propensity score is a conditional probability that a patient will receive a treatment. Covariates described above, including MED, were in the duration model. These same variables, plus opioid use duration, were included in the dose model. After obtaining the propensity scores, we applied inverse probability of treatment weighting using standard approaches.27–29 Weighting resulted in 2 pseudopopulations within each source of patient data, 1 for duration and 1 for dose. In the weighted data, survival models are not confounded by factors that predict exposure, as detailed elsewhere.27–30
Analysis
Before weighting data, we computed bivariate analyses including analysis of variance and t tests for continuous variables and χ2 tests for categorical variables. Bivariate analyses were recomputed after weighting to determine that variables balanced across levels of opiate use duration and MED. Observation time was defined as month of depression diagnosis since start of the follow-up period or right-censoring time. Right-censoring time is month of last known visit relative to the start of the follow-up period. Hazard ratios for time to new-onset depression were estimated using Cox proportional hazards models in which opioid use duration and MED were time-dependent variables. Fully adjusted Cox models included additional, time-dependent control variables for painful conditions and, in the VHA cohort, pain scores to account for change in pain after opioid initiation. The PHREG procedure in SAS 9.4 (SAS Institute), with α set at .05, was used for the Cox regression models. Evaluation of hazard trends over time confirmed that proportional hazard assumptions were met for both duration (P = .33, P = .29, P = .08 in VHA, BSWH, HFHS, respectively) and dose (P = .62, P = .36, P = .13 in VHA, BSWH, and HFHS, respectively). Two-tailed tests were conducted to allow for both risk factors and protective effects. This project was approved by the institutional review boards of participating institutions.
RESULTS
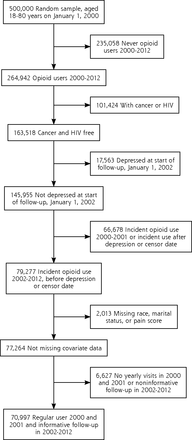
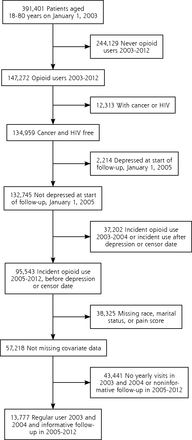
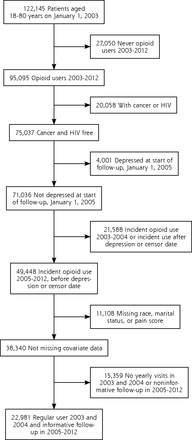
After excluding patients with missing data on any covariate, there were 70,997 VHA, 13,777 BSWH, and 22,981 HFHS patients who were opioid-naive and free of depression at the start of follow-up (Figures 1, 2 and 3). VHA patients were on average older, predominately male, and had a higher percentage of longer than 90 days of opioid use compared with private sector patients. The distributions of MED and other patient characteristics by health system are shown in Table 1.
Eligibility criteria for Veterans Hospital Administration patient population.
HIV = human immunodeficiency virus.
Eligibility criteria for Baylor Scott & White patient population.
HIV = human immunodeficiency virus.
Eligibility criteria Henry Ford Health System patient population.
HIV = human immunodeficiency virus.
Distribution of Opioid Exposure and Covariates by Health Care Organization
Among VHA patients, 11.6% of 1- to 30-day users, 13.6% of 31- to 90-day users, and 14.4% of longer than 90-day users had new-onset depression. Among BSWH patients, cases of new-onset depression increased from 8.4% of 1- to 30-day users to 10.6% of 31- to 90-day users to 19% among longer than 90-day users. In HFHS patients, cases of new-onset depression increased from 10.7% in 1- to 30-day users to 14.8% in 31- to 90-day users to 19.3% in longer than 90-day users.
New-onset depression was significantly associated with MED in VHA patients (P <.0001) but not in BSWH and HFHS patients. In VHA patients new-onset depression increased from 11.9% among those using 1 to 50 mg/d of MED to 14.2% in those using 51 to 100 mg/d of MED to 20.1% in those using greater than 100 mg/d of MED. In BSWH patients new-onset depression occurred in 8.7% of those using 1 to 50 mg/d of MED, in 7.7% of those using 51 to 100 mg/d MED, and in 9.6% of those using greater than 100 mg/d MED. In HFHS patients new-onset depression occurred in 11.3% of those using 1 to 50 mg/d of MED, in 10.8% of those using 51 to 100 mg/d of MED, and in 12.4% of those using greater than 100 mg/d of MED.
The bivariate relationships between opiate analgesic users, covariates, and new onset of depression are available in Supplemental Appendix 1, Table 1, available at http://www.annfammed.org/content/14/1/54/suppl/DC1. In each patient sample, opioid use duration and covariates were significantly associated with a new onset of depression. The bivariate associations between new-onset depression and covariates and duration of opioid use are shown in Supplemental Appendix 1, Table 2, and the bivariate associations of these variables by MED are available in Supplemental Appendix 1, Table 3. Propensity scores models and inverse probability of treatment weighting were successful in removing significant associations between covariates and opioid use duration and dose. Balanced covariate distributions are shown by opioid use duration (Supplemental Appendix 1, Table 4) and dose (Supplemental Appendix 1, Table 5). The prevalence of back pain across opioid use duration in the BSWH patient sample did not balance; however, this variable is included in multivariate survival models.
As shown in Table 2, before weighting data (model 1), VHA patients who were 31- to 90-day users were significantly more likely to develop new-onset depression (hazard ratio [HR] = 1.23; 95% CI, 1.16–1.31) compared with 1- to 30-day opioid users. Similar results were found in BSWH and HFHS patients. Compared with 1- to 30-day opioid use, longer than 90 days of opioid use was significantly associated with new-onset depression in each patient sample.
Association Between Duration of Incident Opioid Use and New-Onset Depression, Unweighted and Weighted by Inverse Probability Duration Exposure
In all 3 patient samples, after weighting data and adjusting further for potential persistent painful conditions (see model 3), 31 to 90 days of opioid use, compared with 1 to 30 days of opioid use, was significantly associated with new-onset depression (HR range = 1.18–1.33), and opioid use longer than 90 days, compared with 1 to 30 days, was significantly associated with new-onset depression (HR range = 1.35–2.05). Hazard ratios obtained for risk from 31 to 90 days and longer than 90 days of opioid use were significantly different from each other in VHA and HFHS patient samples.
As displayed in Table 3, before balancing variables in model 1 and without covariate adjustment, higher MED was associated with a new onset of depression. There were no significant associations between MED and new-onset depression in BSWH and HFHS patient samples before data weighting (model 1). After weighting data and additional adjustment for pain in model 3, and also for pain scores in VHA data, MED was not significantly associated with new-onset depression in VHA, BSWH, and HFHS patients.
Association Between Last Daily Morphine Equivalent Dose of Incident Opioid Use and New-Onset Depression, Unweighted and Weighted By Inverse Probability Dose Exposure
DISCUSSION
In 3 separate diverse patient populations, longer duration of opioid analgesic use was associated with episodes of new-onset depression after controlling for pain conditions and daily MED. Hazard ratios increased from 1.18 (VHA) to 1.33 (HFHS) for 31- to 90- day opioid use to a range of 1.35 (VHA) to 2.05 (HFHS) for longer than 90 days or opioid use; these point estimates are significantly different from each other in VHA and HFHS. We observed that higher MED (greater than 100 mg/d) compared with lower daily MED (1 to 50 mg/d) was not associated with new-onset depression in models controlling for pain and opioid use duration. This finding is not consistent with our previous study, which found that increased MED is associated with greater probability of new-onset depression. It is possible that increasing the MED was a proxy for duration in our previous report.15
Research on the efficacy of opioids in depression treatment, limited by small samples, short follow-up time, and lack of control groups, does not support opioids as effective long-term treatments for depression.31 This evidence, combined with the finding from the present study, supports the conclusion that opioids may cause short-term improvement in mood, but long-term use is associated with risk of new-onset depression.
Limitations
Duration of opioid use did not account for intermittent opioid administration by patients and frequency of dose escalation. In preliminary analysis of VHA patient data, we found no evidence of a dose-by-duration effect and no evidence that risk increased with longer use at higher doses. The report of pain is generated in the clinical setting. Thus, self-report bias is a possible limitation, because patients seeking opioids and those with poor mood may report more pain. We did not have statistical power to model use of longer than 180 days in private sector data. We speculate that risk would continue to increase with longer durations, because our prior study of VHA data found that longer than 180-day opioid use compared with less than 90-day use was associated with new-onset depression (HR = 1.51; 95% CI,1.31–1.74).14
New-onset depression may have developed during or after opioid use cessation. Post hoc analysis in VHA data indicates 93.2% of cases of a new onset of depression occurred after the end of the incident opioid use. Of these patients, 8.7% had new-onset depression begin within 30 days, 8.3% within 31 to 180 days, and 82.9% after 180 days postincident opioid use. The mean lag time between end of incident use and new-onset depression was 3.4 years, (SD = 2.5 years). This lag time may be an overestimate, because symptoms of depression often begin well before patients seek treatment and receive a diagnosis.
The current body of research contributes some evidence toward causality32 in that there is consistency of findings for duration of use across samples with substantial variation in demographic and disease profiles, the association is strong, and the temporal order is established. A dose-response relationship, however, does not exist for MED and new-onset depression, and residual confounding, especially in the case of maximum dose, may be present. A contribution of dose to new-onset depression should not be ruled out. Prospective data collection to obtain information on impaired functioning, opioid misuse, and family dysfunction is warranted.
There are several potential mechanisms for our findings. We speculate that long-term opioid analgesic use could lead to hyperalgesia,33 which in turn leads to a new onset of depression. Chronic opioid use may cause changes in neuroanatomy. Opioid analgesic use lasting 9 years vs less than 1 year in humans correlated with changes in functional connectivity in the nucleus accumbens and amygdala, regions associated with mood regulation, impulse control, reward, and motivation.34 Patients using an average of 78 mg of morphine per day had reduced gray matter in amygdala after 30 days of use, with changes persisting for at least 4.7 months after opioid cessation.35 These studies support the hypothesis that opioid exposure leads to neuroanatomical disturbance in brain regions associated with reward and pleasure, which could be the mechanism underlying new-onset depression. Consequences of chronic opioid analgesic use, such as low testosterone and opioid misuse, could also be in the causal pathway to new-onset depression.
Using the hazard ratio found in the VHA patient population, the number of patients exposed to longer than 90 days of opioid analgesic use that resulted in 1 subsequent case of depression during our observation period is 12. Depression is a substantial public health problem given the magnitude of opioid exposure in the United States. The current results highlight additional concerns for primary care physicians of whom many report frustration and substantial burden in provision of optimal pain management.20
Patients should be informed of this association and be monitored for depression. Clinicians should consider the pain-independent contribution of opioid use when depressed mood develops. Further research is warranted to identify which patients are most vulnerable to opioid-related depression.
Acknowledgment
Yong Hu provided HFHS data analysis.
Footnotes
Conflicts of interest: authors report none.
Funding support: This study was supported by the National Institute of Mental Health, Prescription Opioid Analgesics and Risk of Depression, R21MH101389. This work also received support from the Veterans Health Administration.
Disclaimer: Funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The views are the authors and do not necessarily reflect the views of the Department of Veterans Affairs.
Previous publication: Portions of this study were presented to the North American Primary Care Research Group Scientific Meeting, Cancun, Mexico, October, 2015.
Supplementary materials: Available at http://www.AnnFamMed.org/content/14/1/54/suppl/DC1.
- Received for publication June 29, 2015.
- Revision received September 17, 2015.
- Accepted for publication October 19, 2015.
- © 2016 Annals of Family Medicine, Inc.