Abstract
PURPOSE The aim of the study was to test a tailored lifestyle intervention for helping obese primary care patients achieve weight loss and increase physical activity.
METHODS We conducted a 24-month randomized clinical trial in Rhode Island. Primary care physicians identified obese, sedentary patients motivated to lose weight and increase their moderate to vigorous physical activity. These patients were randomized to 1 of 2 experimental groups: enhanced intervention (EI) or standard intervention (SI). Both groups received 3 face-to-face weight loss meetings. The enhanced intervention group also received telephone counseling calls, individually tailored print materials, and DVDs focused on diet and physical activity. Active intervention occurred in year 1 with a tapered maintenance phase in year 2.
RESULTS Two hundred eleven obese, sedentary patients were recruited from 24 primary care practices. Participants were 79% women and 16% minorities. They averaged 48.6 years of age, with a mean body mass index (BMI) of 37.8 kg/m2, and 21.2 minutes/week of moderate to vigorous physical activity. Significantly more EI participants lost 5% of their baseline weight than SI participants (group by visit, P <.001). The difference was significant during active treatment at 6 months (37.2% EI vs 12.9% SI) and 12 months (47.8% vs 11.6%), but was no longer significant during the maintenance phase at 18 months (31.4% vs 26.7%,) or 24 months (33.3% vs 24.6%). The EI group reported significantly more minutes of moderate to vigorous physical activity over time than the SI group (group by visit, P = 0.04). The differences in minutes per week at 6 months was 95.7 for the EI group vs 68.3 minutes for the SI group; at 12 months, it was 126.1 vs 73.7; at 18 months, 103.7 vs 63.7, and at 24 months, 101.3 vs 75.4. Similar trends were found for absolute weight loss and the percentage reaching national guidelines for physical activity.
CONCLUSION A home-based tailored lifestyle intervention in obese, sedentary primary care patients was effective in promoting weight loss and increasing moderate to vigorous physical activity, with the effects peaking at 12 months but waning at 24 months.
INTRODUCTION
Obesity is a public health problem of epidemic proportions affecting more than one-third of the adult US population.1 Obesity increases the risk for multiple medical conditions that reduce quality of life and increase mortality, including hypertension, diabetes, coronary heart disease, stroke, and some cancers.2,3 The economic burden is large and increasing; medical care for obesity-related diseases is estimated to reach $48 billion to $66 billion dollars a year in the United States by 2030.4 Despite numerous efforts to identify successful weight loss strategies, the prevalence of obesity has remained stable.5 Although interventions can produce weight reduction to improve health and delay onset of diabetes and hypertension,6–8 the existing research-based programs have not been translated into clinical practice.
A review of weight loss interventions in primary care shows that while evidence of the effectiveness of weight loss interventions delivered by primary care physicians is limited,9–16 primary care physicians can play a critical role in diagnosing obesity, evaluating changes in weight-related comorbid conditions, and referring patients to trained interventionists.17 Primary care physicians are in a unique position to refer patients because they reach most segments of the population, and their expertise is highly regarded.18 Consistent with this, recent translational weight loss studies using a variety of team-based approaches have shown benefit; these studies, however, have largely focused on weight loss and not concomitant physical activity.18–22 Tailored interventions that match individual patient characteristics with treatment hold promise to be effective for promoting weight loss and increasing physical activity, as well as for their generalizability to clinical application.23–37 Efficacious, individually tailored interventions that can be implemented by print, telephone, video, or a combination of these media without the need for extensive face-to-face or group counseling may be an effective and easy-to-disseminate model that primary care physicians could refer patients to. We evaluated such an intervention in a randomized, controlled trial of obese, sedentary adults recruited from primary care practices.
The primary aim of the Choose to Lose study was to evaluate the effectiveness of a tailored lifestyle intervention, which we will call the “enhanced intervention” (EI), in promoting weight loss and increasing physical activity compared with a standard intervention (SI) for obese patients referred by primary care physicians. We hypothesized that the EI group would demonstrate greater reductions in weight at the end of active treatment (12 months) and better maintenance of these reductions over time (at 24 months) compared with the SI group. In addition, we hypothesized that the EI group would engage in greater levels of moderate to vigorous physical activity at 12 months and maintain a higher level of physical activity at the 24-month follow-up compared with the SI group.
METHODS
Study Design
Choose to Lose was a 2-arm, randomized-controlled translational research trial. The active treatment phase occurred from baseline to month 12, with a tapered maintenance phase during months 13 to 24. Details of the study design and recruitment have been published previously34 with sample materials included in the Supplemental Appendix, available at http://www.annfammed.org/content/14/4/311/suppl/DC1. All study materials and protocols were reviewed and approved by the Institutional Review Boards of Memorial Hospital of Rhode Island and Brown University. Participants gave written informed consent before initiation of the study protocol.
Randomization, Allocation, and Blinding
After the baseline visit was completed, participants were block randomized within practice in pairs using a random number generator created by the data manager with SPSS for Windows, version 11.0 (IBM). After completion of the initial lifestyle counseling session, the research assistant gave each participant an envelope that revealed the study arm to which the participant was assigned. The research assistants, clinic examiners, research statisticians, data entry personnel, primary care providers, and study investigators were all blinded to the allocation. Lifestyle counselors were unblinded after the initial counseling session.
Study Population
We drew study participants from the practices of 24 primary care physicians selected from a total of 41 physicans who had expressed an interest in participating. The 24 physicians represented 24 primary care practices in Rhode Island and southeastern Massachusetts.
Of the 24 physicians, 54% were male, 75% family physicians, 25% general internists, and 83% US medical school graduates; the average age of participating physicians was 53, with an average of 26 years in practice. To enable them to refer patients to the study appropriately, physicians and their staffs underwent a training session that included discussion of patient inclusion and exclusion criteria, the proposed intervention, and study outcome measures.
Participant inclusion criteria included being 18 to 80 years old with a BMI of at least 25 kg/m2 and the ability to read and speak English and provide informed consent. Exclusion criteria included having a family member already enrolled in the study and having a health condition that might make participation in a weight loss and exercise study unsafe. For full eligibility criteria see Hartman et al34 and the Supplemental Appendix (http://www.annfammed.org/content/14/4/311/suppl/DC1).
Patients referred by their primary care physicians were screened by telephone to determine initial eligibility. Of 610 patients screened, 211 met all eligibility criteria and were randomized into the study (Figure 1). A total of 105 participants were randomized to the EI arm and 106 to the SI arm. Four participants were censored after randomization because they had become pregnant the first 12 months of the trial.
CONSORT diagram of participant flow.
Role of Primary Care Physician
The principal role of the primary care physicians was to identify patients motivated to lose weight and increase physical activity and refer those patients to this home-based intervention. During a training session, the physicians were given guidance about providing an environment supportive of weight loss and increasing physical activity, including capitalizing on teachable moments in the visit. The physicians were updated about their patients’ progress during the study to support management of related comorbidities, to give patients further accountability, and to promote adherence to the weight loss and physical activity regimen.
Interventions
The intervention design and materials have been described previously34; sample materials are available in the Supplemental Appendix (http://www.annfammed.org/content/14/4/311/suppl/DC1). Briefly, both the SI and EI groups began with 12 months focused on weight loss and lifestyle changes under the guidance of registered dietitians trained as lifestyle counselors, followed by a 12-month maintenance intervention. All participants met with a lifestyle counselor at baseline and set a weight loss goal of 10% over 6 months. They were given a structured meal plan dependent on their starting weight to support a 500 to 1,000 kcal reduced-calorie diet based on the Diabetes Prevention Program guidelines.35 Participants were encouraged to add 10 minutes of moderate-intensity activity most days of the week and to work up to engaging in 300 minutes of moderate physical activity per week by 6 months. Participants were given food and exercise self-monitoring diaries for the first 6 months. All participants also met with their lifestyle counselor at 6 and 12 months to review progress and set new goals as needed.
Standard Intervention
In addition to the 3 face-to-face meetings with the lifestyle counselors, participants in the SI group received 5 pamphlets (3 in year 1 and 2 in year 2) produced by the National Institute for Diabetes and Digestive and Kidney Diseases on weight loss, physical activity, and healthy eating.
Enhanced Intervention
Participants in the EI group received phone calls from the lifestyle counselor monthly for the first 6 months and bi-monthly for the next 6. For the first 12 months, they also received weekly mailings that included print materials, feedback on food and exercise logs, and 2 exercise-related DVDs. The mailings focused on participant motivation, weight loss, calorie and exercise goal attainment, journal compliance, food-related issues, and comorbid conditions. Four of these mailings were tailored nutrition reports based on information gathered on the counseling calls. Enhanced intervention participants also received monthly tailored exercise feedback reports for the first 12 months focused on processes of change, decisional balance, and self-efficacy. The reports were generated from a computer-based expert system in response to the participant’s answers to monthly questionnaire items.30–32 In the maintenance phase during the second year, EI participants received tailored and non-tailored materials bi-weekly for the first 6 months and monthly for the last 6 months. They also received exercise feedback reports 4 times throughout the second year and 2 nutrition-related DVDs.
Data Collection
Data were collected at baseline and at 6-, 12-, 18-, and 24-month visits by assessors blinded to group assignment. Demographic data collected at baseline included age, race, sex, and insurance status. Height, weight, waist circumference, resting heart rate, and resting blood pressure were measured at each visit. Physical activity was assessed using an interview-administered 7-day Physical Activity Recall Questionnaire.36 To anchor the participant’s perception of moderate intensity activity, he or she performed a 10-minute treadmill walk at a 3–4 mph pace before completing the physical activity recall questionnaire.32
Statistical Analysis
For baseline characteristics, P values were computed with χ2 or exact method if cells were small for the categorical variables and with analysis of variance (ANOVA) for continuous variables. An intention-to-treat, repeated-measurement, mixed-effect model with variance component covariance structure was used for data analysis since block randomization was performed by practice, and participants varied by age, sex, and race within practice. Means of weight are modeled as a function of the group assignment, visit time, and their interaction, and adjusted by age, race, sex, and clinic. Variances were sourced from the participant level and the clinic level. Model-based standard errors were computed for the fixed-effect parameter estimates and functions of them. Point estimates of weight were computed using average age, race, and sex. If weight or physical activity was missing at follow-up, the value from the previous visit was used, following the last-value-carried-forward imputation method. Before the study, a sample size calculation revealed that 104 participants per arm were needed to find a 5% difference in weights between the groups at α = .05, with 80% power, with 3 repeated measures and intra-class correlation of 0.50 for repeated measures. A fourth 18-month measurement was added after recruitment was complete to ensure adequate power given the sample size of 211 participants.
RESULTS
Study Participants
Table 1 shows the baseline demographic and clinical characteristics of the randomized sample. The CONSORT diagram (Figure 1) shows that retention rates differed at 6 months (84% vs 73%), and 12 months (80% vs 70%), with more active participation in the EI group than the SI group, but were similar at 18 months (72% vs 72%) and 24 months (70% vs 70%).
Baseline Characteristics of Study Participants
The average baseline BMI of those who completed the program was less than that of those who didn’t, both at 6 months (37.6 kg/m2 vs 38.1 kg/m2) and at 12 months (37.4 kg/m2 vs 38.9 kg/m2) but was not different between the EI and SI groups, so differential follow-up would not explain our results.
Weight Loss
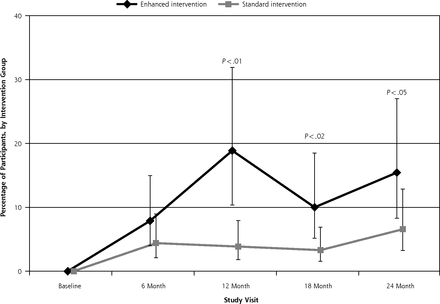
Participants in both the EI and SI groups lost weight at each study visit relative to baseline. Clinically significant weight loss was defined as losing 5% or more of baseline weight. Overall, significantly more EI participants than SI participants lost 5% of their baseline weight (group by visit, P <.001; Figure 2). The difference was significant during active treatment at 6 months (37.2% EI vs 12.9% SI, P <.01) and 12 months (47.8% vs 11.6%; P <.01), but was no longer significant during the maintenance phase at 18 months (31.4% vs 26.7%, P = .64) or 24 months (33.3% vs 24.6%, P = .39).
Percentage of participants attaining 5% weight loss from baseline by intervention group over 24 months.
Similarly the EI group lost significantly more weight overall than the SI group (group by visit, P = .02). During the active intervention phase the members of the EI group lost more weight on average than the members of the SI group both at 6 months (5.0 kg vs 3.4 kg, P = .11) and at 12 months (5.4 kg vs 3.8kg, P = .10), but these differences did not reach statistical significance. The differences were not sustained during the maintenance phase at 18 months (4.4 kg vs 4.3 kg, P = .87) or at 24 months (4.1 kg vs 4.0 kg, P = .89) (Table 2).
Change from Baseline in Weight and Minutes of Moderate and Vigorous Physical Activity per Week Over 24 Months
Physical Activity
Participants in both the EI and SI groups increased their weekly minutes of moderate to vigorous physical activity, with the EI group reporting significantly more minutes over time (group by visit, P = .04). The EI group had more minutes than the SI group at all study visits after baseline, with the difference reaching statistical significance at 12 and 18 months only: 6 months (95.7 vs 67.9, P = .10); 12 months (126.1 vs 73.7, P = .002); 18 months (103.6 vs 63.3, P = .02); 24 months (101.3 vs 75.0, P = .12) (Table 2).
Overall, a low percentage of participants in both groups met the national physical activity guidelines of at least 150 minutes of moderate to vigorous physical activity per week. At 12 months, however, significantly more EI participants than SI participants met guidelines, and the difference remained significant at 18 months and 24 months, although the overall group by visit interaction did not quite reach statistical significance (P = .06) (Figure 3).
Percentage of participants attaining ACSM guideline levels of physical activity by intervention group over 24 months.
ACSM = American College of Sports Medicine.
Intervention Adherence
Both groups had high adherence for the face-to-face visits (2.8 of 3, on average) and for EI, high adherence with the phone calls; on average, 7 out of 8 calls were completed (Table 3). Participants in the EI group mailed in an average of 14 food and exercise journals out of a possible 24 during the first 6 months. On average, EI participants received 3.8 of 4 individually tailored nutrition mailings over the first 6 months, which relied upon information gathered from monthly lifestyle phone calls. The individually tailored physical activity reports required completing a monthly mailed questionnaire and were sent on average 6 out of 13 possible times during year 1 and 1.4 out of 4 possible times during year 2.
Adherence to Behavioral Intervention, by Intervention Group
Adverse Events
Fifty-two adverse events involving 40 different participants occurred, divided nearly equally between the groups: SI = 25, EI = 27. Of the adverse events, 42 were related to musculoskeletal issues, 10 were medical conditions (3 hypoglycemic episodes in participants with diabetes, 1 episode of chest pain, and 1 gallbladder surgery) and 5 were related to symptoms: shortness of breath, light-headedness, etc. Of the 52 adverse events, 21 resulted in referrals to the participants’ primary care physicians, and 3 required medical intervention.
DISCUSSION
This randomized, controlled translational research trial evaluated the effectiveness of a home-based weight loss and physical activity intervention for obese, sedentary adults that was mediated by referrals from primary care physicians. This intervention involved limited face-to-face contacts in combination with weekly tailored print and monthly telephone contacts over the first year, with a tapered maintenance phase during the second year. Almost half of the EI group had lost more than 5% of baseline weight—a clinically significant amount—by the end of the active treatment phase, with one-third maintaining the loss at 24 months. Similar trends were seen for moderate to vigorous physical activity, with the EI group peaking at an average of 126.1 minutes per week at the end of active treatment, with a slight drop to 101.3 minutes per week by the end of the maintenance phase. While clinically significant weight loss of more than 5% was achieved by many participants, the difference between groups in actual weight loss only reached statistical significance for an overall effect but not at any single time point, and the percentage of participants that reached national guidelines for physical activity was modest. The benefits appeared to peak at 12 months for both weight loss and increasing moderate to vigorous physical activity, with no statistically significant difference in benefit between the groups at 24 months.
Other recent trials testing weight loss programs based on referrals from primary care physicians have shown significant weight loss, including studies conducted as part of the Practice-based Opportunities for Weight Reduction (POWER) trials.19–23 Two of those studies21,22 did not show the drop-off in weight loss that was seen in our study in the second year. The intervention arms in both studies, however, continued to have phone calls and/or group sessions in the second year, which our study did not have. This continued contact may be important for weight loss maintenance. Of note, in the current study we were able to achieve about as much weight loss as one of the POWER trials22 without the use of the meal replacements and weight loss medications that study involved. This is important, since meal replacements and medications can be costly to patients, and medications are not without risks, including potential increases in blood pressure, increases in heart rate, and gastrointestinal complaints.37,38 Our study of patients from diverse primary care practices, including many from low socioeconomic populations, showed more beneficial results than the third POWER trial, which demonstrated a modest a 1 kg of weight loss in 3 community health centers.23 That study focused on weight loss and hypertension control in a high risk, socioeconomically disadvantaged population and did not have a robust physical activity component, which may explain their more modest results.
While our trial provides encouraging results regarding this translational weight loss and physical activity intervention, its limitations must be considered. This study was conducted in 1 geographic location, Rhode Island, though it did reach 24 individual practices in its scope. While there remains the possibility of contamination because EI and SI participants were in the same primary care physician’s practice, this would bias our results toward the null. Our sample was predominately female, which might limit generalizability, and our measure of physical activity relied on self-report. In addition, the study used multiple channels of intervention delivery (tailored and untailored print, DVDs, and phone), so we could not reliably assess the individual contributions of these channels. A post-intervention interview with participants suggested that the lifestyle counselors’ personalized goal setting and monthly phone calls were most helpful. Future research should examine which channel of delivery or combination of channels is most effective and focus particularly on the maintenance phase of the study, given the attenuation of results after 12 months. The goal should be not only to determine which channel is most effective but to clarify the optimal frequency of continued contact and whether such contact is better initiated by the patient or the provider team.
The current study contributes to the growing body of research suggesting that weight loss interventions supported by primary care physicians and delivered by third parties have significant benefits for weight loss and, as our results suggest, increasing physical activity.17–20,22 The use of primary care physicians as primary identifiers of patients who are motivated to lose weight adds to the translatability of the Choose to Lose intervention. Our results imply that referral by a primary care physician to a home-based program with limited face-to-face contact can lead to weight loss and increases in moderate to vigorous physical activity. Future iterations of this intervention could use ancillary health care staff in primary care offices or peer counselors in combination with the computerized tailoring software used in this study to provide tailored content. Future research should also examine the use of technology, such as web, e-mails, text messages, or smart phone apps as other avenues to provide support and tailored content. The amount of continued contact and the number of tailored booster sessions needed to support maintenance of weight loss and physical activity needs further research. Future studies should also address Spanish speakers given the growing population in the United States and the high levels of diabetes and obesity in this group. Lastly, future studies should test the cost-effectiveness of such interventions in dissemination trials.
In conclusion, primary care physicians can play a key role in supporting patients’ weight loss efforts by providing referrals to lifestyle programs that incorporate weight loss and physical activity. Home-based, individually tailored weight loss interventions with minimal face-to-face contact can be effective for helping patients reach clinically significant weight loss and increased physical activity goals, realizing that only 25% to 35% of participants will be able to maintain a 5% clinically relevant weight loss at 24 months if our findings are generalizable. Better understanding the amount of and channel for continued contact needed to support maintenance of weight loss and physical activity is needed for the long-term success of obesity and sedentary lifestyle management in primary care.
Acknowledgments
We would like to thank all the patients and the primary care physicians and staff that participated in this study.
Footnotes
Conflict of interest: authors report none.
Funding support: This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (5R18DK079880). Dr Hartman is supported by grant 1K07CA181323 from the National Cancer Institute.
Supplementary materials: Available at http://www.AnnFamMed. org/content/14/4/311/suppl/DC1/.
- Received for publication November 20, 2015.
- Revision received March 11, 2016.
- Accepted for publication March 22, 2016.
- © 2016 Annals of Family Medicine, Inc.