Abstract
PURPOSE Community-acquired pneumonia (CAP), acute cough, bronchitis, and lower respiratory tract infections (LRTI) are often caused by infections with viruses or Streptococcus pneumoniae. The prevalence of atypical pathogens Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, and Bordetella pertussis among patients with these illnesses in the ambulatory setting has not been previously summarized. We set out to derive prevalence information from the existing literature.
METHODS We performed a systematic review of MEDLINE for prospective, consecutive-series studies reporting the prevalence of M pneumoniae, C pneumoniae, L pneumophila and/or B pertussis in outpatients with cough, acute bronchitis, LRTI, or CAP. Articles were independently reviewed by 2 authors for inclusion and abstraction of data; discrepancies were resolved by consensus discussion. A meta-analysis was performed on each pathogen to calculate the pooled prevalence estimates using a random effects model of raw proportions.
RESULTS Fifty studies met our inclusion criteria. While calculated heterogeneity was high, most studies reported prevalence for each pathogen within a fairly narrow range. In patients with CAP, the overall prevalences of M pneumoniae and C pneumoniae were 10.1% (95% CI, 7.1%–13.1%) and 3.5% (95% CI, 2.2%–4.9%), respectively. Consistent with previous reports, M pneumoniae prevalence peaked in roughly 6-year intervals. Overall prevalence of L pneumophila was 2.7% (95% CI, 2.0%–3.4%), but the organism was rare in children, with only 1 case in 1,765. In patients with prolonged cough in primary care, the prevalence of B pertussis was 12.4% (95% CI, 4.9%–19.8%), although it was higher in studies that included only children (17.6%; 95% CI, 3.4%–31.8%).
CONCLUSIONS Atypical bacterial pathogens are relatively common causes of lower respiratory diseases, including cough, bronchitis, and CAP. Where surveillance data were available, we found higher prevalences in studies where all patients are tested for these pathogens. It is likely that these conditions are underreported, underdiagnosed, and undertreated in current clinical practice.
- community acquired pneumonia
- cough
- respiratory tract infection
- Mycoplasma pneumoniae
- Chlamydophila pneumoniae
- Legionella pneumophila
- Bordetella pertussis
INTRODUCTION
Cough is the 4th most common reason for an office visit to an ambulatory physician, accounting for 2.8% of all visits.1 In primary care, when cough is the patient’s primary complaint, it is most often caused by a virus, but approximately 5% of patients have community-acquired pneumonia (CAP).2 Although viruses and Streptococcus pneumoniae are the most common causes of CAP, some episodes are caused by an atypical bacterial infection such as Mycoplasma pneumoniae, Chlamydophila pneumoniae (also known as Chlamydia pneumoniae), and Legionella pneumophila. Some episodes of non-pneumonia lower respiratory tract infection (LRTI) are caused by the above pathogens as well as by Bordetella pertussis, and the incidence of the latter is increasing in the United States.3
Mycoplasma pneumoniae infection is thought to vary cyclically,4,5 and has been the cause of outbreaks of LRTI.6 Not to be confused with Chlamydia psittaci (which also causes respiratory infections but is contracted from birds), Chlamydophila pneumoniae is more common in children, but has been associated with subsequent serious adult disease as well. A meta-analysis reported an association with lung cancer in patients with previous C pneumoniae infections,7 while others have posited an association with development of asthma.8,9 Legionellosis, better known as Legionnaires’ disease, is caused by L pneumophila and is most commonly diagnosed as a cause of CAP in patients over 50 years of age, and more often in men than women. The organism is found naturally in the environment, and the infection is associated with inhalation of aerosolized water from sources such as hot tubs and cooling towers.10 Recently, increased risk of infection with L pneumophila has also been linked to wet, humid weather.11 Bordetella pertussis is highly communicable and is a source of significant morbidity in children and prolonged symptoms in all patients. Although B pertussis is the only atypical pathogen to have a widely available vaccine, the incidence of B pertussis in the United States is increasing, with more cases in 2012 than any year previously since 1955.3
The prevalence of atypical pathogens, particularly in the outpatient primary care setting, has not been previously summarized. B pertussis and L pneumophila are reported by national surveillance systems in many countries, but they are laboratory-based systems that are subject to significant underreporting.12 The prevalence of C pneumoniae and M pneumoniae vary widely in previous studies of patients with CAP.
Because these atypical pathogens do not respond to beta-lactams, may carry a different prognosis, and can cause serious complications in some patients, it is important to understand their prevalence. Therefore, we performed a meta-analysis to describe the prevalence of atypical pathogens among 2 groups: patients with cough, acute bronchitis, or LRTI in the ambulatory setting and patients diagnosed with CAP. We also compared these “real world” prevalences with the prevalences reported by surveillance systems, where available.
METHODS
Literature Review
We searched MEDLINE for prospective studies that reported the results of testing for M pneumoniae, C pneumoniae, L pneumophila, or B pertussis in outpatients with cough, acute bronchitis, or LRTI, as well as among inpatients and outpatients diagnosed with CAP. In order to reflect contemporary prevalences and microbiology, searches were limited to articles where the majority of data was collected after January 1, 2000. We included articles with abstracts written in English and German (the primary languages of the investigators). Supplemental Appendix A (http://www.annfammed.org/content/14/6/552/suppl/DC1) includes detailed search terms used for each strategy. We also reviewed the reference lists for review articles identified by our search, and of any included studies.
We excluded studies of only or predominantly immunocompromised patients, studies of hospital-acquired infections, studies of special or unusual populations (eg, military recruits), studies of acute exacerbations of chronic obstructive pulmonary disease or asthma, and studies of the etiology of bronchiolitis. Further, we excluded studies set in low- or medium-income countries based on Organisation for Economic Cooperation and Development (OECD) criteria; (Supplemental Appendix B, http://www.annfammed.org/content/14/6/552/suppl/DC1) since we felt that they would not reflect the current practice and epidemiology of the United States. We also excluded case-control studies, case reports, case series and retrospective studies, outbreak investigations, and studies that did not use culture, polymerase chain reaction (PCR), serology, or urine antigen testing (for L pneumophila) to identify pathogens.
Data Abstraction
Two investigators reviewed each abstract to identify articles that should be reviewed in full. Any article selected for full review was examined by both investigators. For each included article, study characteristics and data regarding prevalence were abstracted by both authors. For prevalence data, definite and probable cases were included and possible cases were excluded. Any discrepancies were resolved by consensus discussion.
Surveillance Systems
We used surveillance data reported by high-income members of the OECD.13 The most recent complete data available, from 2012, were abstracted by 2 investigators, with any discrepancies resolved by consensus discussion. For each report, we documented the type of surveillance used, number of cases reported, and total population.
Study Quality
A meta-analysis usually uses a standardized tool to assess the risk of bias.14–16 Unfortunately, there are currently no published tools for assessing bias in studies of disease prevalence. To ensure that the studies included in our meta-analysis were of consistent high quality, we only included studies that met the following criteria: they enrolled consecutive patients, did not gather data from a specialized or unusual population, gathered data prospectively, and used diagnostic tests likely to classify patients accurately as having the pathogen in question.
Analysis
We identified 2 groups for the analysis: patients presenting with acute cough illness or lower respiratory tract symptoms and patients diagnosed with CAP. Where studies reported etiology separately for patients with CAP and those with non-pneumonia LRTI, we report these groups separately as well. Pooled prevalence estimates were calculated with random effects model of raw proportions. Statistical analysis was performed in R (version 3.2.2, R Studio Version 0.99.441), including plots of proportions with each pathogen using the metafor procedure.
RESULTS
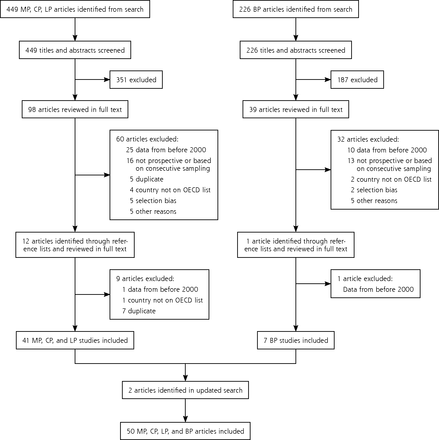
The search for M pneumoniae, C pneumoniae, and L pneumophila yielded 449 abstracts. A separate search for B pertussis returned 226. After screening titles and abstracts, 98 articles for M pneumoniae, C pneumoniae, and L pneumophila and 39 for B pertussis remained for full-text review. Thirteen articles were additionally identified through a review of the reference lists (12 for M pneumoniae, C pneumoniae, and L pneumophila, and 1 for B pertussis). Full-text review excluded 102 articles. The most common reasons for exclusion were that the majority of data was collected before 2000 or that the study did not use a cohort design with prospective data collection. An updated search before writing yielded 2 additional studies17,18 for a final of 50 included studies (Figure 1).
PRISMA diagram.
BP = Bordetella pertussis; CP = Chlamydophila pneumoniae; LP = Legionella pneumophila; MP = Mycoplasma pneumonia; OECD = Organization for Economic Cooperation and Development.
To compare the prevalences given in the identified studies with the prevalences from surveillance systems, we abstracted surveillance data for reported cases of B pertussis and L pneumophila in 2012. Data, which were available for 31 of the 32 high-income member countries of the OECD, are summarized in Table 1 (Israel did not provide any publicly accessible data.)
Reported Bordetella pertussis and Legionella pneumophila Prevalence in 2012 by Case-Based Surveillance Systems of High-Income Countries Belonging to the OECD
Prevalence of Mycoplasma pneumoniae, Chlamydophila pneumonia, and Legionella pneumophila
A total of 30 studies reported the prevalence of M pneumoniae, C pneumoniae, or L pneumophila in adults,18,20–48 and 10 studies reported the prevalence of these pathogens in children49–58 (Table 2). Only 2 studies were set in the United States.18,53
Characteristics of Studies of the Prevalence of Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila in Patients With Community-Acquired Pneumonia or Lower Respiratory Tract Infection
Patients With Community-Acquired Pneumonia
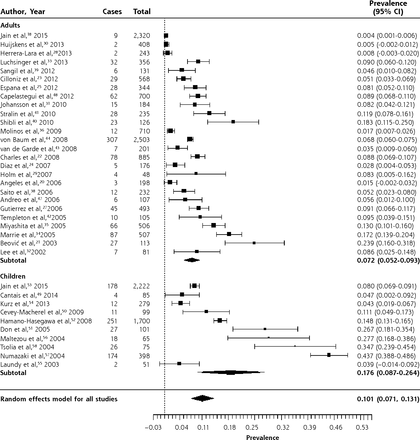
Figures 2–4 show the forest plots for M pneumoniae, C pneumoniae, and L pneumophila respectively in patients with CAP. The overall prevalence of M pneumoniae was 10.1% (95% CI, 7.1%–13.1%). The prevalence was higher in children (17.6%; 95% CI, 8.7%–26.4%) than in adults (7.2%; 95% CI, 5.2%–9.3%). There was significant heterogeneity, though, especially in studies of children. This is likely because outbreaks of M pneumoniae are thought to occur every 4 to 6 years, and inspection of the forest plot, which is sorted chronologically, does reveal peaks around 2004 and 2010.62,63
Forest plot of the prevalence of Mycoplasma pneumoniae in adults and children with community-acquired pneumonia, sorted in reverse chronological order.
Heterogeneity (I2) = 99.27
Forest plot of the prevalence of Chlamydia pneumoniae in adults and children with community-acquired pneumonia, sorted by prevalence.
Heterogeneity (I2) = 98.4
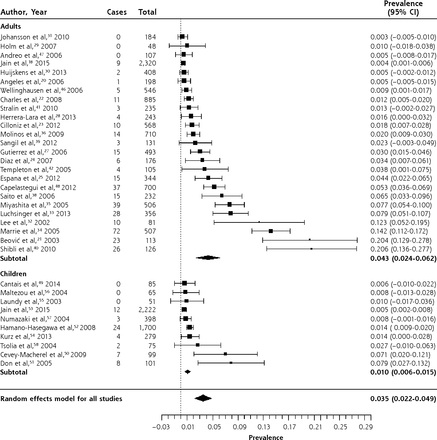
Forest plot of the prevalence of Legionella pneumophila in adults and children with community-acquired pneumonia, sorted by prevalence.
Heterogeneity (I2) = 91.18
The overall prevalence of C pneumoniae in patients with CAP was 3.5% (95% CI, 2.2%–4.9%). Infection with C pneumoniae was more common in adults (4.3%, 95% CI, 2.4%–6.2%) than in children (1.0%, 95% CI, 0.6%–1.5%). There was significant heterogeneity, although only 4 of 25 studies in adults had a prevalence greater than 10%, while the remainder had a prevalence between 0.3% and 7.7%. In children, only 2 of 10 studies had prevalences greater than 5%, while the remaining 8 had prevalences ranging from 0.5% to 2.7%. We reviewed the 6 identified outliers, but were unable to determine a reason for their high prevalence. There was also no clear pattern of variation by year of study.
Legionella pneumophila was exceedingly rare in children, with only 1 case in 1,765 patients with CAP.52,56 The overall prevalence in adults was 2.8% (95% CI, 2.1%–3.6%), although in most studies it was between 1% and 3%. Again, there was significant heterogeneity. Of the studies reporting a prevalence of 5% or higher, 4 of 6 were in Spain,27,28,36,37 and a fifth, a study that also reported the highest prevalence of C pneumoniae, was set in another Mediterranean country, Israel.40 The largest series, set in Germany, found L pneumophila in 3.7% of patients treated in ambulatory care and 3.8% of inpatients.44 Clearly, it is not only found in severely ill patients.
Patients With Non-Pneumonia LRTI
Two studies reported the prevalence of atypical pathogens in patients with LRTI in whom pneumonia had been excluded by normal chest radiography,29,57 and a third enrolled predominantly patients with non-pneumonia LRTI.59 The prevalence of M pneumoniae was 7/316 (2.2%), 13/129 (10.0%), and 78/523 (14.9%) in these 3 studies,29,57,59 while the prevalence of C pneumoniae was 2/316 (0.6%) in 1 study29 and 3/523 (0.6%) in a second.57 A single study found no cases of L pneumophila in a primary care series of 316 adults with non-pneumonia LRTI.29 A fourth study did not provide adequate information to differentiate the number of children with acute bronchitis, pneumonia, or bronchiolitis.60
Prevalence of Bordetella pertussis in Outpatients
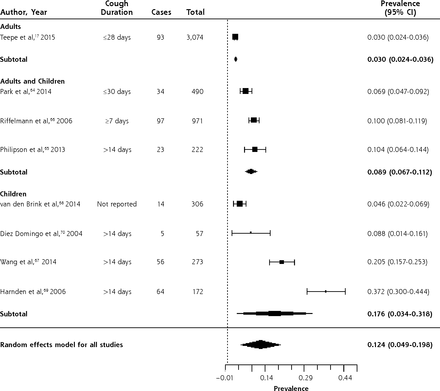
Table 3 summarizes data from 8 studies of the prevalence of B pertussis in outpatients with prolonged or bothersome cough, largely in primary care.17,64–70 Three studies enrolled adults and children; 4, children only; and 1, adults only. Data were collected between 2001 and 2012. One study assessed children referred from primary care due to suspicion for B pertussis, based on the duration of cough.68 The prevalence of B pertussis is summarized in the forest plot in Figure 5. While there was significant heterogeneity when including all studies, this was primarily due to heterogeneity in the 4 studies of children only.
Characteristics of Studies of the Prevalence of Bordetella pertussis in Outpatients With Prolonged Cough or Non-Pneumonia Lower Respiratory Tract Infection
Forest plot of the prevalence of Bordetella pertussis in outpatients with prolonged cough or non-pneumonia lower respiratory tract infection, sorted by prevalence.
Heterogeneity (I2) = 98.83
The overall prevalence was 12.4% (95% CI, 4.9%–19.8%). In a large, multi-country, European prospective study of adults presenting to primary care with cough of up to 28 days duration,17 prevalence was 3% (95% CI, 2.4%–3.6%). The prevalence was higher in studies of children (17.6%; 95% CI, 3.4%–31.8%) than in those of adults and children (8.9%; 95% CI, 6.7%–11.2%), but there was significant heterogeneity in the studies of children, with a range from 4.6% to 37.2%.67–70
Surveillance Data for Bordetella pertussis and Legionella pneumophila
Of the 26 countries to report data on B pertussis, Australia had the highest incidence rate of 105.0 cases per 100,000 persons per year. Hungary reported the lowest incidence rate of 0.05 cases per 100,000 persons per year. With 48,277 cases, the United States had the most reported cases of all countries, twice as many as the next country. Of the 30 countries reporting L pneumophila, the United States had the most cases at 3,688. Poland reported the lowest incidence of L pneumophila (0.02 per 100,000 persons per year) and Slovenia the highest (4.02 per 100,000 persons per year). It is likely that differences in surveillance systems and reporting account for much of this variability.
DISCUSSION
Among adults with CAP, 14% had an atypical pathogen: 7% had Mycoplasma pneumoniae, 4% had Chlamydophila pneumoniae, and 3% had Legionella pneumophila. Among children with CAP, 18% had Mycoplasma pneumoniae, only 1% had Chlamydophila pneumoniae, and Legionella pneumophila was extremely rare (1 case in 1,765 patients). Among patients with prolonged cough, 9% of adults and 18% of children had Bordetella pertussis.
Evidence for Underdiagnosis
CAP is diagnosed in an estimated 5.6 million patients annually in the United States, and 1.1 million hospitalizations result.71,72 Laboratory-based surveillance, however, identifies only 3,700 infections caused by L pneumophila each year, or 0.06% of all community-acquired pneumonias. Our systematic review found that when a consecutive series of patients with CAP are all tested for L pneumophila, it is detected in 3% of patients, with a range of 1% to 10%. This is consistent with the most recent US study,18 which found that 1.9% of episodes of CAP in a consecutive series of hospitalized adults were caused by L pneumophila. If 2% of all episodes of CAP are caused by L pneumophila, this would be 112,000 cases per year. Thus, the vast majority of cases of L pneumophila in the United States, approximately 100,000, may be undiagnosed. It is therefore important that physicians consider this pathogen when diagnosing CAP, and consider ordering urine antigen tests for L pneumophila more routinely, particularly when patients are non-responsive or slowly responsive to therapy with a beta-lactam. The recommended antibiotic for L pneumophila is a respiratory fluoroquinolone.73,74
Similarly, the annual incidence of acute bronchitis or non-pneumonia LRTI is approximately 440 episodes in 10,000 adults,75 and the annual incidence of B pertussis based on surveillance is 1.5 of 10,000 persons. Our systematic review found that 18% of episodes of non-pneumonia LRTI in children and 9% of those in adults were caused by B pertussis. Most of these studies limited inclusion to patients with a cough for at least 1 to 2 weeks, although 1 included adults and children with a shorter duration of cough and still found a prevalence of 7%.64 If one conservatively estimates based on these data that 3% of episodes of acute bronchitis or non-pneumonia LRTI are caused by B pertussis, that corresponds to 13 episodes per 10,000. Again, these data suggest that there is widespread underdiagnosis of B pertussis in the United States, with approximately 90% of episodes undiagnosed. This is important because family members and relatives are the source for 75% to 83% of pertussis cases in infants.76,77 Moreover, immunization with the pertussis vaccine wanes after five years.78–80 Current recommendations to vaccinate pregnant women with Tdap should be closely adhered to.
C pneumoniae infection has traditionally been described as being more common in children. We found that the mean prevalence, however, was 4% in studies of adults with CAP compared with 1% in children.
Diagnosis of these infections could be improved in several ways. One is to make better use of the history and physical examination. The best evidence regarding diagnosis of each pathogen is summarized in Table 4. Data regarding diagnosis are quite limited, and only in the case of L pneumophila has an attempt been made to develop and validate a clinical decision rule that combines several signs and symptoms.84 In general, individual signs and symptoms are of little value in the diagnosis of these atypical pathogens. Another approach would be to integrate signs and symptoms with a point-of-care test such as c-reactive protein (CRP), as has been done for pneumonia and influenza diagnosis.86,87 Greater use of urine antigen tests for L pneumophila should be encouraged for patients diagnosed with CAP, and the development of accurate, rapid point-of-care tests for C pneumoniae and B pertussis should be prioritized.
Accuracy of Signs and Symptoms for Respiratory Infections With Atypical Pathogens
Limitations
As with any systematic review, our conclusions are limited by the quality of the published literature and the completeness and accuracy of reporting. We found considerable heterogeneity. For M pneumoniae this may be related to the cyclical nature of outbreaks, while for other pathogens the cause is less clear but may may lie in the differences in the populations studied, varying laboratory techniques, and varying sample collection methods across countries. It is noteworthy that the majority of studies found similar prevalences, with the heterogeneity for C pneumoniae and L pneumophila introduced by a small number of outliers, and for B pertussis limited to studies in children only. We limited our analysis to studies that gathered data within the past 15 years in highly developed economies, so our findings may not be generalizable to low- or middle-income countries. Many patients with acute cough do not seek care. It is possible that those seeking care have a different (and perhaps more severe) illness and a different prevalence of these pathogens. Finally, the literature regarding the prevalence of pathogens in patients with non-pneumonia lower respiratory tract infection is quite limited, with no studies in the United States or Canada.
We have demonstrated that atypical bacterial pathogens are relatively common causes of CAP in a range of populations including both adults and children, and that B pertussis is a common cause of prolonged cough. We do not feel that broader use of antibiotics for patients with acute cough is warranted. What is needed are studies to help clinicians more accurately diagnose these pathogens or to help them identify a large group of patients at low risk for such pathogens who do not require further testing or antibiotic therapy. Approaches that develop clinical decision rules integrating signs, symptoms, and point-of-care tests such as CRP are particularly promising.88 Finally, research is needed to determine if and when antibiotics are helpful, since data regarding treatment of B pertussis and M pneumoniae from well designed, adequately powered contemporary clinical trials are lacking.
Footnotes
Conflicts of interest: authors report none.
Supplementary materials: Available at http://www.AnnFamMed.org/content/14/6/552/suppl/DC1/
- Received for publication February 5, 2016.
- Revision received June 2, 2016.
- Accepted for publication July 13, 2016.
- © 2016 Annals of Family Medicine, Inc.