Abstract
PURPOSE Polypharmacy is a key clinical challenge for primary care. Drugs that should be prescribed for an intermediate term (longer than 3 months, but not indefinitely) that are not appropriately discontinued could contribute to polypharmacy. We named this type of prescribing legacy prescribing. Commonly prescribed drugs with legacy prescribing potential include antidepressants, bisphosphonates, and proton pump inhibitors (PPIs). We evaluated the proportion of legacy prescribing within these drug classes.
METHODS We conducted a population-based retrospective cohort study using prospectively collected data from the McMaster University Sentinel and Information Collaboration (MUSIC) Primary Care Practice Based Research Network, located in Hamilton, Ontario. All adult patients (aged 18 or older) in the MUSIC data set during 2010-2016 were included (N = 50,813). We calculated rates of legacy prescribing of antidepressants (prescription longer than 15 months), bisphosphonates (longer than 5.5 years), and PPIs (longer than 15 months).
RESULTS The proportion of patients having a legacy prescription at some time during the study period was 46% (3,766 of 8,119) for antidepressants, 14% (228 of 1,592) for bisphosphonates, and 45% (2,885 of 6,414) for PPIs. Many of these patients held current prescriptions. The mean duration of prescribing for all legacy prescriptions was significantly longer than that for non–legacy prescriptions (P <.001). Concurrent legacy prescriptions for both antidepressants and PPIs was common, signaling a potential prescribing cascade.
CONCLUSIONS The phenomenon of legacy prescribing appears prevalent. These data demonstrate the potential of legacy prescribing to contribute to unnecessary polypharmacy, providing an opportunity for system-level intervention in primary care with enormous potential benefit for patients.
- polypharmacy
- bisphosphonates
- antidepressive agents
- proton pump inhibitors
- family practice
- primary health care
- health services research
- electronic health records
- potentially inappropriate medication list
- inappropriate prescribing
- practice-based research
INTRODUCTION
Inappropriate polypharmacy is a key clinical challenge for primary care and has been well described.1-3 Concerns about polypharmacy relate to its potential effects on quality of life due to adverse drug reactions, including both direct drug effects (eg, falls, impaired cognition, and poorer nutrition) and drug interactions. Polypharmacy is also associated with reduced medication adherence and difficulties in managing complicated medication regimens that exceed patients’ ability to cope.4-9
In Canada, adverse drug reactions cause an estimated 70,000 preventable hospital admissions per year.4 Adverse drug reactions requiring medical care affect a substantial proportion of older adults (occurring in 13% of those on 5 or more medications); one-third are considered preventable.10,11
Drivers of polypharmacy, and current strategies to reduce inappropriate medication use and polypharmacy, along with evidence for their effect, are reviewed elsewhere.3 Single-disease guidelines have been flagged as unsuitable for use in patients with multimorbidity and just one driver of polypharmacy3,12,13; however, other systemic aspects of care and prescribing that contribute to inappropriate polypharmacy are less clear.
Inappropriate prescribing is often conceptualized in drug-based terms: the total numbers, types, or combinations of drugs concurrently pre scribed, for example, as outlined by Beers Criteria or anticholinergic burden.14-16 It can also be conceptualized in terms of prescribing duration. Intermediate-term prescribing may be thought of as prescribing that is indicated for more than 3 months but usually not indefinitely. Inappropriate prescribing can occur when, despite initial appropriateness, these drugs are not discontinued after their usual effective or recommended period. We have termed this legacy prescribing and hypothesize that it may represent a substantial issue, contributing to inappropriate polypharmacy.
Primary care serves a coordinating function for patients with multimorbidity and is also the setting for most long-term prescribing, making this an appropriate setting to study and address inappropriate medicine use and polypharmacy. We investigated the extent of legacy drug prescribing for 3 exemplar drug classes prescribed for different conditions—antidepressants, bisphosphonates, and proton pump inhibitors (PPIs)—using routinely collected electronic health record prescribing data within a primary care practice-based research network (PBRN).
METHODS
Study Design and Setting
We undertook a retrospective cohort study using prospectively collected electronic health record data from January 2010 through December 2016. The study was conducted within the McMaster University Sentinel and Information Collaboration (MUSIC) PBRN in Hamilton, Ontario. Patients served by the MUSIC network represent a wide range of socioeconomic statuses, from distributed neighborhoods within Hamilton and the surrounding area. Practitioners were 60% female, and the mean year of medical school graduation was 1994.
Data Source
The MUSIC data set comprises aggregate, deidentified electronic health record data extracted quarterly and contributed to the Canadian Primary Care Sentinel Surveillance Network (CPCSSN) data set, which is used to describe the epidemiology of primary care in Canada (REB Project Number 14-731). Each new MUSIC data extract is checked and is compared with previous extracts to ensure integrity and stability. From this database, we extracted medications and patient demographics for patients aged 18 to 100 years as of December 31, 2016, as the data set for analysis. Anatomic Therapeutic Chemical (ATC) codes were used to identify specific prescribing data for the 3 drug classes of interest: antidepressants, bisphosphonates, and PPIs (Supplemental Table 1, available at http://www.annfammed.org/content/16/6/515/suppl/DC1/).
Legacy Prescribing Case Definition
Legacy prescriptions were identified by calculating prescription durations for each drug class. Our definitions for legacy status for each class were conservative to ensure that this status represented considerable inappropriate exposure. We selected evidence-based inclusion criteria, as follows.
For antidepressants, we used a continuous prescribing duration of longer than 15 months. Treatment is recommended for 6 months after resolution of an acute mood episode and is then stopped in most cases.17 Stopping before 6 months results in a higher relapse rate.18
For bisphosphonates, we used a continuous prescribing duration of longer than 5.5 years. Treatment for osteoporosis is recommended for up to 5 years in most cases, as the beneficial effect persists beyond 5 years if the medication is stopped at this point.19,20
For PPIs, we used a continuous prescribing duration of longer than 15 months. Evidence supports only short-term use (for less than 1 year) of PPIs in most cases.21
Data Integrity and Legacy Prescribing Calculation
The MUSIC database contains a complete set of all prescriptions written by PBRN practices for their enrolled patients and contains data on prescription product, dose, duration, and date. We removed any nonprescribing data appearing as a prescription (for example, notes to the pharmacist), as well as individual prescriptions with erroneous duration values of more than 2,000 days or those with end dates after 2018. All remaining individual prescriptions for each drug class were grouped per patient.
Few studies have described methods for extraction and analysis of medication data from primary care electronic health records, in particular for prescribing duration analysis.22-26 In the absence of a standardized approach, we pragmatically developed and tested 2 methods for assessing prescribing duration. Prescription duration using these 2 methods was derived for each patient with prescriptions for the drug classes of interest. One measure, sum duration, was calculated by summing the difference (in days) between the start and stop dates of each prescription, grouped per patient, per drug class. The other measure, start-stop duration, was derived from the difference between the first-ever start date and the last-ever stop date for each drug class grouping per patient.
Legacy Prescribing Case Validation
To determine the most accurate duration measure, we applied the legacy criteria to patients’ sum duration and start-stop duration value pairs, and coded patients to 1 of 4 categories per drug class of interest: (1) legacy based on sum duration only; (2) legacy based on start- stop duration only; (3) legacy based on both sum duration and start-stop duration; or (4) nonlegacy based on absence of both duration criteria. Next, the raw prescribing data from a random selection of patients from each of the 4 categories were examined to confirm or refute the legacy assignment and gauge the accuracy of each duration method.
For start-stop duration, we checked for any considerable time gaps in continuous prescribing series of 6 months or longer for PPIs and antidepressants and 1 year or longer for bisphosphonates. Where we detected discontinuous prescribing, we calculated new durations for continuous prescriptions. For values no longer satisfying legacy criteria, we noted the patient’s legacy group assignment as inaccurate (these prescriptions may, for example, represent appropriate intermittent prescribing for recurrence or relapse).
We examined the sum duration data to detect any anomalies in the recording of prescriptions. We found duplicate prescriptions, overlapping prescriptions, and prescriptions of 0 days in duration that were contributing to inaccuracy in sum duration measures.
The data validation process demonstrated that the sum duration method was compromised by certain data-recording inaccuracies and the start-stop duration method was unreliable when intermittent prescribing occurred in a prescribing series. In comparison, the start-stop duration method was reasonably accurate for detecting legacy prescribing in a prescription series, but was more robust when the associated sum duration value also satisfied legacy criteria. This validation step confirmed that the most correct estimate of legacy prescribing for a given drug class is when both sum duration and start-stop duration criteria are satisfied, and we therefore used this definition in our study. Similarly, only patients who did not meet criteria for both sum duration and start-stop were considered true nonlegacy patients in this study. All patients satisfying only sum legacy criteria or only start-stop legacy criteria were included in the denominator of total patients ever prescribed the drug class, but were left out of all subsequent study analyses comparing characteristics of legacy prescription and nonlegacy prescription patient groups. (Supplemental Table 2, available at http://www.annfammed.org/content/16/6/515/suppl/DC1/provides the detailed validation data and statistical analyses.)
We calculated the proportion of current patients who still had active legacy prescriptions at the end of the study period (January 1, 2017) among patients with an active status in the electronic health record on December 31, 2016.
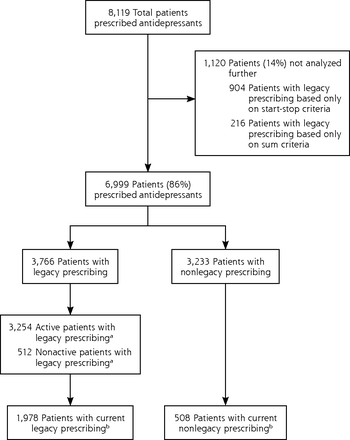
Figure 1 illustrates this delineation of legacy and nonlegacy prescription patients for the antidepressant drug class, including subsets defined by active patient status and current drug prescriptions. We applied this same sorting technique to the other 2 drug classes to arrive at their subsets for analyses.
Patients prescribed antidepressants: inclusion and exclusion for comparative analysis.
aPatients designated with an active status in their patient record as of December 31, 2016.
bPatients had both an active status and a current prescription for the drug associated with legacy prescribing status (stop date after December 31, 2016).
RESULTS
Analyses were based on 50,813 adult patients. Table 1 outlines the summative counts and proportions of patients prescribed drugs within each of the 3 drugs classes, and shows whether prescribing was legacy or not. The overall proportion of legacy vs nonlegacy prescribing was fairly evenly split: 43% and 44%, respectively. The mean proportion of prescribers’ patients receiving legacy prescription was 10% (25th percentile = 7%, 75th percentile = 12%; interquartile range = 5%).
Legacy Prescriptions Among Patients Prescribed Each Index Drug Class During 2010-2016
The pooled total number of patients with legacy prescriptions across the 3 drug classes (6,879) amounted to 5,806 unique patients (10% among all 50,813 patients within the PBRN data set for analysis), indicating that certain patients received legacy prescriptions in more than 1 class. Table 2 shows the patterns of single and dual legacy prescriptions for these patients. Of these unique patients, 17% (969 of 5,806) had legacy prescriptions for both antidepressants and PPIs. No other drug class combination showed notable dual legacy status.
Legacy Prescription and Coprescription: Unique Patients With Single- or Multiple-Drug Legacy Prescriptions, 2010-2016
A large proportion of patients who experienced legacy prescribing during the study period still had current active prescriptions for these medications at the end of the study period (61% of those for antidepressants, 65% of those for PPIs, 77% of those for bisphosphonates) (data not shown). A greater proportion of women received legacy prescriptions for antidepressants and bisphosphonates, but not for PPIs, compared with respective men prescribed the same drug classes (Supplemental Table 3, available at http://www.annfammed.org/content/16/6/515/suppl/DC1/). Across all drug classes, patients with legacy prescribing were relatively older than counterparts with nonlegacy prescribing.
DISCUSSION
Main Study Findings
Our findings suggest that legacy prescribing is prevalent, is consistent across prescribers, and could be an important system-level contributor to inappropriate polypharmacy. The high proportion of currently active legacy prescriptions found represents an opportunity for both research and improvement in patient care.
The high level of coprescription of antidepressants and PPIs may represent an important and previously unreported prescribing cascade, whereby the adverse effects of an index medication mimic the symptoms of a disorder, for which another medication is then prescribed.27 Selective serotonin reuptake inhibitors, which are the most commonly prescribed antidepressant class, have substantial gastrointestinal effects, supporting this potential association.
Strengths and Limitations
We used routinely collected data from a PBRN to provide valuable practice-based evidence on longitudinal prescribing patterns in a real-world primary care setting.28 With no validated standard methodology previously described in the literature, we pragmatically developed and validated one that reasonably estimates prescribing duration. This method can be applied and refined in other health care settings to define rates of legacy drug prescribing, serving as a useful canary in the coal mine signal of systemic prescribing issues.
There are important limitations linked to the nature of the data. Although many appropriate intermittent prescriptions will have been excluded by requiring both start-stop and sum legacy criteria to be filled, a certain proportion of prescriptions meeting these criteria will be clinically appropriate given specific patient characteristics, and that determination was beyond the scope of this study. For example, some guidelines suggest longer-duration antidepressant therapy (up to 2 years or longer) for certain limited patient subgroups. This recommendation, though weak (opinion-based Strength-of-Recommendation Taxonomy = C) and not supported by evidence of effectiveness in the primary care population, may explain some of the effect seen.17,29-36 Nonetheless, the data presented indicate long-term prescribing levels and mean durations far exceed what might be expected.
Of the 3 drugs classes studied, we found comparatively fewer legacy bisphosphonate prescriptions, possibly because the duration of the cohort data available is close to the legacy duration definition for this drug class. Future availability of longitudinal data will clarify this finding.
Finally, the data we used represented prescribing and not dispensing, so they likely overestimate patient exposure. On the other hand, this study did not include other concurrent, specialist-prescribing data outside of the MUSIC PBRN, potentially leading to an underestimate of legacy prescribing.
Study Implications
Legacy prescribing appears to be an important contributor to inappropriate prescribing. Although noted in theory as a prescribing fault, inappropriate duration has been largely invisible as a source of inappropriate prescribing. This invisibility may occur because studies of error are largely undertaken in secondary care settings and because drug-based assessments are used.37-39 Prescribing systems are largely geared toward starting and continuing medicines; most have no controls to flag the end of an intermediate-term prescription, while systems and software features for routine prescription refilling are common. Our results are therefore not surprising and indicate a need for system-oriented change that encompasses prescribing systems, education, and patient-pharmacist-physician communication on appropriate stopping of drug therapy.39,40
Labor-intensive audit and feedback solutions are often workarounds for system flaws, and can only discover when potentially inappropriate prescribing has already occurred. We suggest the best timing for interventions will be at initial and any repeated prescription, aiming to preempt legacy prescribing with patients as an essential partner in shared decision making.
There is fertile ground here to improve prescribing and reduce unnecessary overtreatment, polypharmacy, morbidity, and costs associated with adverse drug reactions.12,41 Legacy prescribing could also be explored as a quality measure incentivizing restraint in a system where there are few, if any, current indicators of the adverse effects of too much medicine.42
Acknowledgments:
The authors would like to thank the primary care clinicians and patients of the MUSIC PBRN who contribute their data to the network through which the study data were generated; Krzysztof Adamczyk, the IT lead for the MUSIC network; and the support of the McMaster University Department of Family Medicine for this PBRN.
Footnotes
Funding support: Funding for this research was provided by the Pilot Research Project Fund, Department of Family Medicine, McMaster University.
Conflicts of interest: authors report none.
To read or post commentaries in response to this article, see it online at http://www.AnnFamMed.org/content/16/6/515.
Previous presentations: Mangin D, Lawson J, Cuppage J, et al. Legacy drug prescribing patterns and associations in primary care: cohort study in the MUSIC. Poster presented at the Trillium Research Day Conference; May 31, 2017 practice based research network; Toronto, Ontario, Canada.
Mangin D, Lawson J, Cuppage J, et al. Legacy drug prescribing patterns and associations in primary care: cohort study in the MUSIC practice based research network. Poster presented at the NAPCRG Annual Conference; Nov 19, 2017; Montreal, Quebec, Canada.
Mangin D, Lawson J, Cuppage J, et al. Legacy drug prescribing patterns and associations in primary care: cohort study in the MUSIC practice based research network. Oral presentation at the NAPCRG Practice-based Research Network Conference; June 26, 2018; Bethesda, Maryland.
Supplemental Materials: Available at http://www.AnnFamMed.org/content/16/6/515/suppl/DC1/.
- Received for publication March 23, 2018.
- Revision received August 27, 2018.
- Accepted for publication September 10, 2018.
- © 2018 Annals of Family Medicine, Inc.