Abstract
PURPOSE To test the efficacy of intra-articular hypertonic dextrose prolotherapy (DPT) vs normal saline (NS) injection for knee osteoarthritis (KOA).
METHODS A single-center, parallel-group, blinded, randomized controlled trial was conducted at a university primary care clinic in Hong Kong. Patients with KOA (n = 76) were randomly allocated (1:1) to DPT or NS groups for injections at weeks 0, 4, 8, and 16. The primary outcome was the Western Ontario McMaster University Osteoarthritis Index (WOMAC; 0-100 points) pain score. The secondary outcomes were the WOMAC composite, function and stiffness scores; objectively assessed physical function test results; visual analogue scale (VAS) for knee pain; and EuroQol-5D score. All outcomes were evaluated at baseline and at 16, 26, and 52 weeks using linear mixed model.
RESULTS Randomization produced similar groups. The WOMAC pain score at 52 weeks showed a difference-in-difference estimate of −10.34 (95% CI, −19.20 to −1.49, P = 0.022) points. A similar favorable effect was shown on the difference-in-difference estimate on WOMAC function score of −9.55 (95% CI, −17.72 to −1.39, P = 0.022), WOMAC composite score of −9.65 (95% CI, −17.77 to −1.53, P = 0.020), VAS pain intensity score of −10.98 (95% CI, −21.36 to −0.61, P = 0.038), and EuroQol-5D VAS score of 8.64 (95% CI, 1.36 to 5.92, P = 0.020). No adverse events were reported.
CONCLUSION Intra-articular dextrose prolotherapy injections reduced pain, improved function and quality of life in patients with KOA compared with blinded saline injections. The procedure is straightforward and safe; the adherence and satisfaction were high.
- intra-articular hypertonic dextrose
- knee osteoarthritis
- normal saline
- prolotherapy
- randomized clinical trial
INTRODUCTION
Knee osteoarthritis (KOA) is ubiquitous in primary care, leading to pain, disability, and substantial patient and societal costs.1,2 Conservative care is limited. While exercise and weight reduction are effective, the degenerative nature of osteoarthritis and the difficulty of behavioral change appears to limit success.3 Recent studies have confirmed that paracetamol (acetaminophen) confers minimal benefit.4,5 Although nonsteroidal anti-inflammatory drugs are effective, their safety profiles remain a significant concern.6 Other options such as physical therapy, acupuncture, and herbal treatments have marginal effectiveness.7 Other conservative measures, including intra-articular corticosteroid and hyaluronic acid offer short-term benefit but have safety limitations.8 Total knee replacement is effective, but is costly and carries operative risk.9 Therefore, identification of safe and effective nonsurgical therapy remains a high priority in clinical practice and orthopedic research.10
Prolotherapy is an injection therapy used to treat chronic painful musculoskeletal conditions, including KOA.11,12 While various injectants have been used, hypertonic dextrose has been used since the 1950s, is the most common injection used, and is the most studied. Hypertonic dextrose prolotherapy (DPT) has historically been understood to facilitate healing and subsequent pain control through tissue proliferation potentially mediated by an inflammatory mechanism.12
The standard prolotherapy injection protocol involves a whole joint approach with both intra-articular injections into synovial spaces and extra-articular injections at soft tissue bony attachments.13,14 Improvements in knee pain and function have been reported in random-ized controlled trials (RCTs),15,16 systematic reviews and meta-analyses.17,18 However, the independent contributions of the intra-articular vs extra-articular injections is not known. The standard procedure is uncomfortable because of the multiple injections required, and premedication with opioid analgesics has been used.15 In addition, the extra-articular injection protocol requires specialized training not typically received in conventional medical education. These factors may limit patient access to prolotherapy.
Positive effects of an abbreviated protocol (intra-articular injection alone) have been reported, though studies are limited by poor design, small sample size, or lack of a control therapy.19-23 Primary care providers are well positioned to perform an abbreviated technique, given that intra-articular knee injections are straightforward to perform and safe. We therefore conducted a 1-year blinded RCT to assess the efficacy of a brief intervention version of DPT.
METHODS
The Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee (approval number 2014.059) approved this study. Written informed consent was obtained from all participants in the study. The detailed methodology has been reported.24
Study Participants and Setting
We enrolled patients from the general outpatient clinics in the New Territories East region of Hong Kong from February 2016 through October 2017. The inclusion criteria were: age 45–75 years; diagnosis of KOA based on clinical and radiographic criteria as defined by the American Rheumatology College25; moderate to severe knee pain for at least 3 months, defined as a score of ≥3 (on a 0–6-point ordinal scale) in response to the question “What is the average level of your left/right knee pain in the past 3 months?”; and failure to achieve a reduction to less than 3 points, using the same pain scale, after 6 months of conservative care. The exclusion criteria included: corn allergy26,27; previous knee replacement surgery; pregnancy; body mass index ≥35; current anti-coagulant therapy; knee injections within the previous 3 months; a diagnosis of inflammatory or post-infectious knee arthritis, gouty arthritis, psoriatic arthritis, or septic arthritis; significant effusion as defined by a ballotable patella; and comorbidity or lifestyle factors precluding participation in the study.
Randomization, Allocation, Concealment, and Blinding
Blocked randomization (1:1) was undertaken by an off-site statistician using Random Allocation Software to allocate patients to the DPT group or the normal saline (NS) group.28 The randomization sequence was concealed using the sequentially numbered, opaque sealed envelope procedure.29 The envelopes were kept by a person uninvolved in participant care or evaluation, or in the data analysis. Blinding ceased when the envelopes were opened at 52 weeks.
Two registered nurses not involved in participant care and independent of the trial prepared the syringes containing DPT or NS. The dextrose and saline solutions were odorless and identical in color; syringes were wrapped in aluminum foil to mask viscosity. The principal investigator, study coordinator, and the practitioner who performed the injections were blind to treatment allocation. Participants were blinded to treatment group status, knowing only their randomization group number. Research assistants collected all data during face-to-face interviews; the assistants were blind to allocation status. Data entry was performed by personnel external to the research team.
Intervention
Participants were placed in the supine position. Following aseptic preparation and injection of 1 ml of 1% lidocaine HCL (Xylocaine) as a bleb of local anesthetic, the study injection was administered under ultrasound guidance (using a linear probe and in-plane approach) with a 25-gauge needle directed to the suprapatellar pouch to ensure injection into the joint space. If both knees were painful, the more painful knee was injected. Injections were administered at weeks 0, 4, 8, and 16 in both study groups.
The DPT solution comprised 5 ml of 25% dextrose, the concentration commonly used for intra-articular dextrose prolotherapy injection in previous studies.14,15 The solution was prepared by mixing 2.5 ml of 50% dextrose with 2.5 ml of sterile water. Participants in the control group received 5-ml injections of normal saline. Details of post-injection care are in the study protocol.24
Usual care was continued in both groups. Conventional medications, physical therapy, acupuncture, herbal medicines, over-the-counter drugs, and other active treatments were discouraged but allowed and tracked during the study period. All participants were asked to avoid other injection therapies during this time.
Outcome Measures
Outcome measures were assessed at baseline (week 0) and at 16, 26, and 52 weeks except for EuroQol-5D that was assessed at half year intervals (weeks 0, 26, and 52). The primary outcome was the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC; 0-100 points) pain score at 52 weeks, considered the gold standard of self-reported measures in KOA trials.30,31 The minimal clinical important difference of the WOMAC composite score has been reported as 12 points or more for rehabilitation interventions for osteoarthritis.32 A validated Chinese version of the WOMAC was used in this study.33 The secondary outcomes were: the WOMAC function, stiffness, and composite score subscales; 3 objective physical function measures recommended for use in KOA trials (the 30-second chair stand performance test, the 40-meter fast-paced walk test, and the timed up and go test).34 The EuroQol-5D questionnaire was used to measure health-related quality of life.35 The visual analogue scale score in response to the question “What is your average pain score upon walking in the past 48 hours?” was recorded as a unidimensional pain measurement on a 0–100-mm scale. Seven-day recall diaries were used to calculate the number of participants who had used analgesic medications in the previous week. To assess the success of blinding, participants were asked to guess their group status at 52 weeks. Treatment satisfaction was tested by asking “Would you recommend the therapy to others with knee OA like yours?”
Demographics, body weight, height, duration of knee pain, and prior knee interventions were recorded. Baseline physical activity status was assessed using the International Physical Activity Questionnaire.36 The Kellgren-Lawrence classification system37 was used to grade the severity of KOA on existing knee radio-graphs. Adverse events were recorded at each visit.
Sample Size and Statistical Analysis
Sample size calculation and analysis were reported.24 Briefly, we calculated the sample size based on the response to DPT and NS interventions in 2 RCTs.23,38 The participants in these 2 trials had comparable baseline characteristics. The mean (standard deviation) difference between the WOMAC score at 26 weeks and at baseline was 25.2 (20.3) points for DPT23 and 9.53 (26.6) points for NS.26 Assuming a pooled standard deviation of 26.6, 34 participants in each group had 80% power to detect a significant effect size of 0.70 in a 2-sample t-test with an alpha set at 0.05. Assuming a 10% dropout rate, 76 participants were required.
Baseline characteristics of the 2 groups are presented as mean and standard deviation for continuous variables and as number and percentage for categorical variables. International Physical Activity Questionnaire scores are reported as median and interquartile range. We conducted linear mixed models analysis for both primary and secondary outcomes to investigate significant changes over time following the intention-to-treat principle. A nonlinear relationship over time was commonly observed in various outcomes, therefore, time was treated as a categorical variable to capture the nonlinear relationship. We assumed the outcomes between groups at baseline were equal and reflected by the intercept term. Treatment variable was not part of the model but its interaction with time was still in the model.39 The overall treatment effect of nonlinear relationship was examined by linear mixed models analysis, with time indicator 0 for baseline and 1 for follow-up visits. We used the statistical package IBM SPSS Statistics version 21.0 (IBM Corp) and R version 3.4.3 (Project for Statistical Computing). All statistical tests were 2-tailed with a significance level of 0.05. Maintenance of blinding was analyzed and interpreted using an established procedure.40
Only 2 study participants (both in the NS group) were lost to follow-up by week 52, minimizing the potential for bias and reduction in power due to missing data. Assuming missing data were missing at random, the linear mixed model, which used all available data for the full likelihood optimization, produced results that were comparable with those of other approaches, such as multivariate imputation using chained equations. Therefore, the missing data were left as missing and no imputation method was employed. A stopping rule was applied to participants who underwent total knee replacement or experienced severe adverse events during the study period.
RESULTS
Of the 205 participants considered for inclusion in the trial, 76 met eligibility criteria and were enrolled and randomized into 2 groups containing 38 participants each (Figure 1). All participants completed the baseline questionnaire and were included in the intention-to-treat analysis. Participants adhered to the protocol of 4 planned injections except for 1 DPT participant that missed the last injection. Two participants in the NS group were lost to follow-up.
Study flowchart.
The study participants had a mean age of 63.2 years, 71% were female, 21% were overweight, and 46% were obese. Mean duration of knee pain was 8.9 years, and 75% had a Kellgren-Lawrence severity grade of 2 or 3. Fifty-two percent were minimally active and 42% participated in health-enhancing physical activity (Table 1).
Baseline Demographic and Clinical Characteristics of the Dextrose and Normal Saline Injection Groupsa
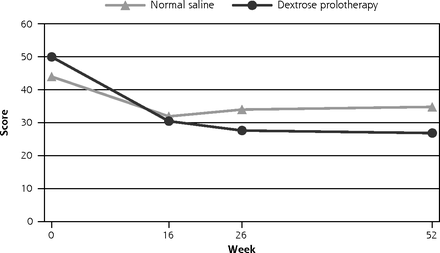
In our primary linear mixed models analysis, all outcomes demonstrated a positive trend favoring the DPT group over the NS group. The WOMAC pain score at 52 weeks showed a difference-in-difference estimate of −10.34 (95% CI, −19.20 to −1.49, P = 0.022).
The improvement was confirmed by the overall trend of −8.26 (95% CI, −14.83 to −1.69, P = 0.014). Similar favorable effect was shown on the difference-in-difference estimate on WOMAC function score of −9.55 (95% CI, −17.72 to −1.39, P = 0.022), the WOMAC composite score of −9.65 (95% CI, −17.77 to −1.53, P = 0.020), visual analogue scale (VAS) pain intensity score of −10.98 (95% CI, −21.36 to −0.61, P = 0.038) and EuroQol-5D VAS score of 8.64 (95% CI, 1.36 to 15.92, P = 0.020). An overall trend of improvement was also observed in the EuroQol-5D index score of 0.09 (95% CI, 0.01 to 0.18, P = 0.037). (Table 2 and Figure 2, Supplemental Appendix 1 and Supplemental Appendix 2, http://www.AnnFamMed.org/content/18/3/235/suppl/DC1/). There were no statistical differences in objectively assessed functional outcomes (Table 2) or medication use (P = 0.350) (Supplemental Appendix 3, http://www.AnnFamMed.org/content/18/3/235/suppl/DC1/). Blinding was successful (Supplemental Appendix 4, http://www.AnnFamMed.org/content/18/3/235/suppl/DC1/). In terms of treatment satisfaction, 94.7% in the DPT group and 91.7% in the NS group reported that they would recommend the treatment to others (P = 0.670). The within-group improvements in the WOMAC composite scores of the DPT and NS groups were 20.9 and 9.4, respectively.
Results of Group Effect on Outcome Measures Using Linear Mixed Modelsa
Change in observed WOMAC pain score from baseline to 52 weeks follow up.
WOMAC = Western Ontario McMaster University Osteoarthritis Index.
Eight serious adverse events were reported during the 52-week study period. Two occurred in the DPT group and 6 in the NS group. None were related to study interventions. One participant in each group underwent a total knee replacement between 26 and 52 weeks.
DISCUSSION
This randomized controlled trial assessing intra-articular DPT for symptomatic KOA found statistically significant improvement in the DPT group compared with NS group on the primary outcome of WOMAC pain score at 52 weeks. Beneficial effects were also demonstrated in WOMAC function, WOMAC composite, VAS pain intensity, and EuroQol-5D VAS and index scores. The change was clinically meaningful, with the composite WOMAC score improvement in the DPT group exceeded the minimal clinical important difference of 12 points at 52 weeks.32 No procedure-related adverse events were reported; adherence to and satisfaction with the treatment protocol was high.
Both the DPT and NS groups demonstrated an overall improvement of WOMAC composite score from baseline. Recent studies have reported that NS serves as an active control instead of a true placebo in KOA injection trials.41 One hypothesis for this mechanism of action is the dilution of inflammatory mediators within the knee, providing relief of perceived pain and subjective stiffness; the potential of a biologic disease-modifying effect of NS cannot be excluded.41,42 Unlike the DPT group, however, the overall improvement in the WOMAC composite score in the NS group was less than the predefined minimal clinical important difference on the WOMAC for KOA trials.32
The DPT group also reported significant improvement of the EuroQol-5D VAS score at 52 weeks; the overall trend of improvement in the EuroQol-5D index also exceeded its minimal clinical important difference of 0.07 points in patients with KOA.43 EuroQol-5D is a well-known quality of life measurement with fair responsiveness of improvement.43 Therefore, the improved EuroQol-5D score in the DPT group suggests a potential global effect of this knee-specific intervention.
Our findings are comparable to the standard whole-joint intra-articular and extra-articular approach. In Rabago’s study,15 the difference-in-difference estimates between the DPT and the NS group were −6.8 in the WOMAC pain score and −10.8 in the WOMAC function score. While we were able to achieve similar pain and functional improvement, the intra-articular approach is simpler and less painful.15
Our findings are also consistent with other studies that have tested an intra-articular DPT protocol for KOA. Reeves et al reported an improved VAS pain intensity score at 6 months.19 Eslamian et al and Topol et al also reported a reduction in the WOMAC pain score at 6 and 9 months, respectively.20,21 However, direct comparisons between studies are limited by heterogeneity of study eligibility criteria, use of different comparison groups, overall health status of patients, baseline severity of KOA, and dextrose concentrations used.
Our findings are also comparable with those of other intra-articular injection therapies for KOA. Intra-articular corticosteroid is known to provide short-term pain relief up to 4 weeks, but clinically important benefits after 1 to 6 weeks remain unclear.44 Therefore, coriticosteroid use is usually indicated for acute inflammatory flares. Intra-articular injection of hyaluronic acid appears to have longer pain relief with peak effects at 8 weeks, which then diminishes to a barely detectable effect by 24 months.45
Intra-articular platelet-rich plasma is an emerging therapy but high-quality scientific evidence of efficacy is still lacking.46 The positive and enduring effect of DPT (to 52 weeks) in this trial suggests DPT be considered as a treatment option in KOA. Future direct comparisons with these therapies will help to define their priority in clinical practice.
The mechanism of action of dextrose in prior studies of prolo-therapy for KOA has been debated but remains unclear in the absence of substantive tissue level basic science. The clinical result may be from multifactorial effects and be associated with both the physical injection procedure and biologic effects of the injectant. Initial clinical and basic science studies of DPT suggest potential mechanisms include stimulation of the inflammatory cascade,47 a non-inflammatory proliferant effect,48-50 and even chondrogenesis.51
There are few limitations of this study. The lack of a usual care group, which is often an exercise group in KOA trials, may limit the external validity, though studies report that DPT is superior to exercise.15,16 We also excluded participants with morbid obesity, defined as body mass index (BMI) ≥35 kg/m2 in the Asian population, which may potentially limit the generalizability to this population. Therefore, we conducted a moderation analysis to explore whether baseline BMI affected the treatment result. We found that there is no effect of BMI on pain outcomes in our study population (Supplemental Appendix 5, available at http://www.AnnFamMed.org/content/18/3/235/suppl/DC1/). The treatment of only 1 painful knee instead of both may not reflect the overall efficacy of DPT in real world practice. The study did not track the amount of exercise and weight loss in each group throughout the year, which may have an influence on the outcomes. Language and culture differences also limited direct comparisons to other work.
Intra-articular DPT injections reduced pain, improved function and quality of life compared with NS injections; the beneficial effects endured through 52 weeks. No adverse events were reported. The adherence and satisfaction to the procedure were high. The outcomes associated with the current abbreviated protocol compares well with those of the more complex standard protocol. The single intra-articular injection is easy to learn, is part of conventional medical training, and is quick and inexpensive. While longer-term follow-up, direct comparison with other injection therapies, cost-effective analysis, and a better understanding of mechanism are needed, the current study suggests that intra-articular DPT may be appropriate care for patients with KOA refractory to more conservative care.
Acknowledgments
The authors would like to thank Miss Lyan LY Chow for administrative tasks, data collection, and data entry; and Miss Lucia WY Tam for nursing support. We thank Dr Julian CY Fong, the radiologist from the Hong Kong College of Radiologists, for reporting the radiographs. The University of Wisconsin Prolotherapy Education and Research Laboratory supports Dr Rabago in collaborative efforts (https://www.fammed.wisc.edu/prolotherapy/).
Footnotes
Conflicts of interest: authors report none.
To read or post commentaries in response to this article, see it online at http://www.AnnFamMed.org/content/18/3/235.
Author contributions: All participated in the conception and design of the study. D.C.C.C. and B.H.K.Y. were responsible for data acquisition and analysis. R.W.S.S., D.R., D.K.R., and S.Y.S.W. interpreted the results of analyses. R.W.S.S., R.W., and D.R. drafted the manuscript. All authors critically revised the manuscript and approved the final version. R.W.S.S. and D.R. are the guarantors. R.W.S.S. attests that all listed authors meet authorship criteria and none meeting the criteria have been omitted.
Funding support: The study was funded by the Chinese University of Hong Kong Direct Grant for Research 2013-14 (HKD 40,000). The funding body had no role in the study other than funding.
Transparency declaration: The lead author R.W.S.S. affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Data sharing: All data in this study are available upon request.
Trial registration: The trial (ChiCTR-IPC-15006617) was registered on the Chinese Clinical Trials Registry on June 17, 2015. (http://www.chictr.org.cn/showprojen.aspx?proj=11247)
Supplemental materials: Available at http://www.AnnFamMed.org/content/18/3/235/suppl/DC1/.
- Received for publication May 29, 2019.
- Revision received October 23, 2019.
- Accepted for publication November 4, 2019.
- © 2020 Annals of Family Medicine, Inc.