Abstract
PURPOSE Adults with a triple multimorbidity (hypertension, prediabetes or type 2 diabetes, and overweight or obesity), are at increased risk of serious health complications, but experts disagree on which dietary patterns and support strategies should be recommended.
METHODS We randomized 94 adults from southeast Michigan with this triple multimorbidity using a 2 × 2 diet-by-support factorial design, comparing a very low-carbohydrate (VLC) diet vs a Dietary Approaches to Stop Hypertension (DASH) diet, as well as comparing results with and without multicomponent extra support (mindful eating, positive emotion regulation, social support, and cooking).
RESULTS Using intention-to-treat analyses, compared with the DASH diet, the VLC diet led to greater improvement in estimated mean systolic blood pressure (−9.77 mm Hg vs −5.18 mm Hg; P = .046), greater improvement in glycated hemoglobin (−0.35% vs −0.14%; P = .034), and greater improvement in weight (−19.14 lb vs −10.34 lb; P = .0003). The addition of extra support did not have a statistically significant effect on outcomes.
CONCLUSIONS For adults with hypertension, prediabetes or type 2 diabetes, and overweight or obesity, the VLC diet resulted in greater improvements in systolic blood pressure, glycemic control, and weight over a 4-month period compared with the DASH diet. These findings suggest that larger trials with longer follow-up are warranted to determine whether the VLC diet might be more beneficial for disease management than the DASH diet for these high-risk adults.
INTRODUCTION
Adults with hypertension, prediabetes or type 2 diabetes, and overweight or obesity are at increased risk of health complications, including stroke, end-stage renal disease, myocardial infarction, premature death, and death from COVID-19.1-4 Separately, each of these conditions is prevalent; nearly one-half (47%) of adults in the United States have hypertension,5 approximately one-half have prediabetes or type 2 diabetes,6 and approximately 42% of US adults are obese.7
Evidence suggests the first-line treatment for these adults should be diet and lifestyle interventions, but experts disagree on which diet should be recommended.8-11 The Dietary Approaches to Stop Hypertension (DASH) dietary pattern is rich in fruits, vegetables, whole grains, and low-fat dairy foods, restricts saturated and total fat, and is lower in sodium. A DASH diet is the standard-of-care dietary recommendation for blood pressure (BP) control by the American Heart Association.10 Another promising diet is a very low-carbohydrate (VLC) diet, also known as a ketogenic or “keto” dietary pattern, which is a very low-carbohydrate, moderate protein, higher-fat diet. A VLC diet has been found to decrease BP,12,13 and it is recommended as an option for glycemic control and weight loss by the American Diabetes Association.14 No studies to date have directly compared a DASH vs VLC diet for efficacy in improving measures of hypertension, diabetes, and weight loss in this population.
In addition, there is limited evidence for the efficacy of behavioral strategies to support dietary adherence and self-management for these 2 diets. Standard behavioral treatment components include support for self-regulation including self-monitoring, goal setting, and providing personalized feedback.15,16 Other emerging approaches for self-management include skills training for the following: (1) mindful eating,17 (2) positive emotion regulation,18 (3) social support,19,20 and (4) cooking education.21,22
In the Michigan’s Hypertension, Diabetes, and Obesity Education Research Online (MHERO) trial, we randomized adults with this triple multimorbidity using a 2 × 2 diet-by-support factorial design, comparing a VLC diet vs a DASH diet, as well as comparing results with and without multicomponent extra support (mindful eating, positive emotion regulation, social support, and cooking), thus including the following 4 groups: VLC, VLC + support, DASH, DASH + support. We hypothesized that both dietary approaches and the extra supports would improve patient outcomes.
METHODS
We conducted the MHERO randomized trial in the United States (NCT03729479); this study was approved by the University of Michigan Institutional Review Board (HUM00146610). Recruitment began in January 2019, and data collection concluded in August 2020. All participants provided informed consent before participating.
Inclusion criteria included age 21 to 70 years, glycated hemoglobin (HbA1c) ≥5.7% within the previous 12 months, a body mass index of 25 to 50 kg/m2, ability to engage in light physical activity, and a systolic BP (SBP) of ≥130 mm Hg as measured in our laboratory using an average of 3 measurements with an Omron Professional IntelliSense Blood Pressure Monitor (HEM-907XL; Omron Healthcare Inc). We asked that all participants take their hypertension medication as normal before arriving at our laboratory.
Exclusion criteria included the following: current use of insulin, phenytoin, lithium, steroids, immunosuppressant drugs, or warfarin; severe renal or hepatic insufficiency; cardiovascular dysfunction (such as congestive heart failure, heart arrythmias, and valvular heart disease); uncontrolled psychiatric disorder; current cancer treatment; pregnant or planning to be within 12 months; weight-loss surgery; vegan or vegetarian; currently enrolled in a formal weight-loss program (such as WeightWatchers); taking weight-loss drugs; untreated thyroid condition; and consumption of >30 alcoholic beverages per week.
We randomized participants to 1 of 4 groups using a 1:1:1:1 ratio. We stratified by body mass index (<30 kg/m2 or ≥30 kg/m2) and sex (male or female). All outcome assessors were blinded to randomization.
All participants received access to a weekly, 4-month online program (with 16 weekly sessions), similar to our previous research,23 with text messages, mailed cookbooks, e-mail–based coaching at least every 2 weeks, and encouragement for all participants to self-monitor their nutritional intake, weight, and BP, and encouragement to self-monitor blood glucose if taking glucose-lowering drugs that might increase the risk of hypoglycemia. We included recommendations for physical activity and sleep hygiene beginning at week 6.
Participants in the VLC groups were encouraged to eat a VLC diet, decreasing their carbohydrate intake to 20 to 35 nonfiber g of carbohydrates a day, with the goal of achieving nutritional ketosis defined as a positive urine dipstick result (Ketostix; Bayer Diabetes Care). We encouraged participants to test ketones at least weekly.24 Participants in the DASH groups were encouraged to follow the DASH diet, limiting sodium to <2,300 mg daily and fat intake to 20% to 30% of calories per day.25 Participants were recommended to eat a variety of fruits and vegetables, lean meats and fish, whole grains, and low-fat dairy.
Participants in the extra support groups were provided information related to the following topics: (1) mindful eating skills including awareness of hunger and fullness sensations, awareness of triggers related to emotional eating, skills to help participants recognize but not act on food-related urges, and mindful responses to stress;17 (2) positive emotion regulation topics including noticing and savoring positive events, positive reappraisal, and gratitude. These skills are based on the positive pathways to health theoretical model18 and the hedonic theory of behavior26 and might facilitate coping and adherence; (3) social support topics including strategies for sharing health information and effectively using one’s social network. Previous research shows that seeking out and sharing health information is associated with lower SBP19; (4) food preparation topics including cooking skill basics and how to build flavor from sweet, salty, bitter, sour, and umami. Lack of cooking skills and confidence are barriers to achieving and maintaining dietary changes.21,22
Measurements
We assessed physical and physiologic measures at baseline and within 1 month of completing the 16th week of the intervention (post). Participants received a $100 Amazon gift card for completing postintervention measures. Fifteen participants (16%) were enrolled when the COVID-19 pandemic began, preventing the completion of some in-laboratory measures, and we had to stop recruitment with 51 potential participants in process, becausee the laboratory was temporarily closed.
Participants were asked to measure their BP at home at least once a week using a BP cuff (Omron 5 or 10; Omron Healthcare Inc). Given the white-coat effect, in which in-office BP can be quite elevated compared with home-measured BP,27 we used the home-based measurement as our primary outcome. Baseline BP was an average of any measurements participants took for the first 2 weeks of the intervention, and outcome BP was an average of any measurements for the last 2 weeks.
Baseline and postintervention HbA1c values were analyzed by the Michigan Diabetes Research Center Chemistry Laboratory. For participants completing the study during the COVID-19 pandemic, we used a mail-in test (DTILaboratories, Inc) for their post HbA1c value.
All participants received a body weight scale (BodyTrace, Inc). Baseline and postintervention body weight were measured in our laboratory, or for participants in the trial during the COVID-19 outbreak, via this home-based scale.28,29
We assessed medications via self-report. We measured program satisfaction with the question, “How would you rate your overall satisfaction with the program?” rated from 1 = not at all satisfied to 7 = very satisfied.
To assess dietary adherence, staff conducted three 24-hour dietary recalls over a period of 1 week at postintervention, which we averaged. To assess adherence to the DASH diet, we created a DASH-adherence score based on the DASH recommendations for whole grains; vegetables; fruits; low-fat foods; nuts, seeds, and legumes; meat, poultry, and fish; sweets; fats and oils; and sodium. The score ranged from 0 to 90, with a higher score indicating greater adherence. In addition, we assessed total daily net (nonfiber) g of carbohydrates. We considered participants to be adherent to the DASH diet if postintervention, they had a DASH score of ≥40 based on prior research,30 and to be adherent to the VLC diet if postintervention, they reported eating ≤90 g of daily g of net carbohydrates.
Analyses
We prespecified change in SBP as our primary outcome and change in HbA1c and percent weight as our secondary outcomes. For our primary analyses, we conducted linear mixed regression models first using an intention-to-treat (ITT) analysis (n = 94). To examine the robustness of findings, we conducted linear regression models for participants with complete data (n = 68 for SBP, n = 81 for HbA1c, and n = 82 for weight). The ITT analysis makes use of all available data at all time points, with the 3 outcomes as dependent variables, and time (pre, post), diet, and support allocation in a full-factorial design with all main effects and interaction terms. For each of the diet and support allocation factors, we report the pre-post change within each of the levels as well as a difference between these changes (interaction with time). A random subject intercept was used to account for within-subject clustering over time. The models were further adjusted for sex and age. For the complete-case analyses, involving participants with pre-post data, a change score was calculated by subtracting postintervention values from baseline values for each outcome. For weight, we also examined percent weight change. Each of these outcomes was used as the dependent variable in a linear regression model with diet, support, and their interaction as primary factors. The models were further adjusted for age and sex. Model diagnostics were carried out using residuals. For all other outcomes, data are shown as mean (SD) or n (%) unless otherwise stated. Analyses were conducted using IBM SPSS 28.0 (IBM Corp).
RESULTS
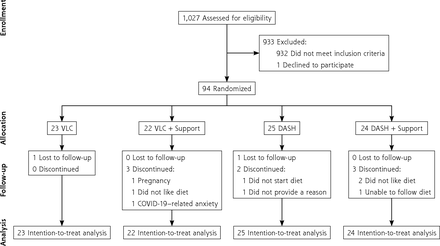
As shown in the Consolidated Standards of Reporting Trials enrollment flow diagram (Figure 1), 94 participants were randomized. Demographic characteristics of the randomized sample are presented in Table 1.
Participant CONSORT flow diagram.
CONSORT = Consolidated Standards of Reporting Trials; COVID-19 = coronavirus disease 2019; DASH = Dietary Approaches to Stop Hypertension; VLC = very low-carbohydrate.
Baseline Participant Characteristics
Table 2 presents results from the ITT analyses, with the β coefficients and SE values from the linear mixed models in Table 3. None of the outcomes of the ITT analyses showed a significant diet × support × time interaction or a significant support × time interaction. We also conducted analyses for completers alone using change scores for each outcome, and none of the analyses showed a significant diet × support interaction or a significant main effect of support. In Table 2, we report results for the main effect of diet within each group and the difference in these main effects across groups (diet × time interaction). However, given that we were also interested in the effect of support, we further present those results in Table 4.
Estimated Mean (SE) of Outcomes Across Diet and Time From Linear Mixed Model
Beta Coefficients and SE Values From Linear Mixed Model
Estimated Mean (SE) of Outcomes Across Support Groups and Time From Linear Mixed Model
In the ITT analyses, SBP decreased more in the VLC group, a difference between the groups of −4.59 mm Hg (P = .046) (Table 2). Results were similar in the completers analysis; SBP decreased by 9.92 (SE, 1.76) mm Hg (n = 33) in the VLC group and by 4.49 (1.70) mm Hg (n = 35) in the DASH group, which was a statistically significant difference between the groups of −5.43 (2.41) mm Hg (P = .028).
In the ITT analyses, HbA1c decreased more in the VLC group, a statistically significant difference between the groups of −0.21% (P = .034) (Table 2). Results were similar in the completers analysis; HbA1c decreased by 0.34 (SE, 0.08) % (n = 39) in the VLC group and by 0.14 (0.07) % (n = 42) in the DASH group, which was a difference between the groups of −0.20 (0.20) % (P = .058).
In the ITT analyses, weight decreased more in the VLC group, a statistically significant difference between the groups of −8.81 lb (P = .0003) (Table 2). Results were similar in the completers analysis; weight decreased by 19.90 (SE, 1.63) lb (n = 41) in the VLC group and by 11.80 (1.66) lb (n = 41) in the DASH group, which was a statistically significant difference between the groups of −8.09 (2.29) lb (P = .001). Percent weight decreased by 8.94 (SE, 0.67; n = 41) in the VLC group and by 4.94 (0.69; n = 41) in the DASH group, which was a statistically significant difference of percent weight between the groups of −4.00 (0.95; P < .001).
As described above and in Table 4, results were not significantly different by whether participants were assigned to the extra support group, although the nonsignificant results were lower (more improved) in the support groups. Results were similar in the completers analyses.
The interaction of diet × support × time was not statistically significant for the ITT analyses for SBP, but given that it was close to significant (P = .06), we explored it in more depth. Systolic BP decreased by 11.15 (SE, 2.17) mm Hg (P = < .001) in the VLC group without extra support, by 8.39 (2.50) mm Hg (P < .001) in the VLC group with extra support, by 2.20 (2.28) mm Hg (P = .34) in the DASH group without extra support, and by 8.17 (2.32) mm Hg (P < .001) in the DASH group with extra support. Therefore, it appears that extra support had some effect in conjunction with DASH diet on reduction of SBP.
Changes in drug regimens from baseline to postintervention are shown in Table 5. At baseline, 72 participants (76.6%) were taking ≥1 BP-lowering drug, and some discontinued or decreased these drugs; 31.3% in the VLC group, 43.8% in the VLC + Support group, 13.0% in the DASH group, and 5.3% in the DASH + Support group. At baseline, 24 participants (25.5%) were taking ≥1 glucose-lowering drug, including glipizide, sitagliptin, and metformin, and some discontinued or decreased these drugs; 40.0% in the VLC group, 75.0% in the VLC + Support group, and none in the DASH and DASH + Support groups. Dietary adherence at postintervention was 14/22 (63.6%) in the VLC group, 15/19 (78.9%) in the VLC + Support group, 18/23 (78.3%) in the DASH group, and 16/21 (76.2%) in the DASH + Support group.
Drug Regimen Changes for Participants Taking Drugs During Trial
Program satisfaction for all groups was high, with 94% of participants rating their satisfaction at or above the midpoint of the scale, and 73% rating themselves at the highest or second-to-highest level of satisfaction. Dropout was low, with 89% of participants completing post measures. There were no treatment-related serious adverse events.
DISCUSSION
In this study, we found that for adults with overweight or obesity, hypertension, and prediabetes or type 2 diabetes, a VLC diet showed greater improvements in SBP, glycemic control, and weight over a 4-month period compared with a DASH diet, although both dietary approaches improved outcomes. To our knowledge this is the first trial to compare these 2 dietary patterns in a population of adults with this high-risk set of metabolic conditions.
The addition of extra support did not have a statistically significant effect on outcomes, although the nonsignificant changes were lower (more improved) in the support groups. Thus, the extra support could have been helpful, but the trial might have been statistically underpowered to detect changes. It might also be that the support given in our standard program was sufficient. In addition, the VLC diet had stable, clinically significant effects on BP regardless of the additional psychosocial support; however, the effects of the DASH diet were dependent on the extra support, suggesting that the DASH diet might need to be integrated with psychologic support to induce clinically meaningful reductions in BP, similar to findings of other low-fat dietary interventions. It is also possible that the method of delivery for the support information was not sufficient to show an additive effect above the standard program. Of note, recent guidelines from the US Preventive Services Task Force recommend more intensive interventions with interaction with a clinician, and our e-mail–based coaching might not have been sufficiently intensive.31
Our primary outcome in this study was exploratory, given that interventions using these combinations of recommendations and supports had not been previously tested. Therefore, this trial was not intended to be powered to find statistically significant differences, and the number of participants recruited reflects the available sample size. That said, with regard to the diet main effects, we found statistically significant outcomes, thus the study was not underpowered with regard to those. It is possible that the study was underpowered with regard to the extra support parameter and the interaction of that with diet. The effect size and variability estimates from this study could help inform larger studies that would be powered to detect differences in the extra support parameter.
The present results contribute to growing evidence regarding the effect of VLC and DASH dietary patterns on metabolic outcomes. For example, meta-analyses suggest that low-carbohydrate diets, especially a VLC diet, are more effective in decreasing hypertension than low-fat diets12,13 and that a VLC diet is also effective for glycemic control and weight loss.32 However, a systematic review and network analysis of 13 dietary approaches found that a DASH diet was the most effective for decreasing BP,33 and an umbrella review of systematic reviews found that a DASH diet also decreases body weight and improves glycemic control.34 In a meta-analysis, researchers found that across 30 randomized controlled trials of a DASH diet in adults with and without hypertension, the DASH diet decreased SBP by 3.2 mm Hg more than a variety of different comparison diets.35 In our trial, the estimated mean difference in decrease between the VLC and DASH groups for SBP was 4.6 mm Hg in the ITT analysis, an even greater difference, and in the opposite direction; the VLC diet showed greater improvement than the DASH diet.
Our trial had several limitations. One is that we had to create our own definition of dietary adherence because there is no standard for this. Another was that we did not provide participants food; therefore dietary adherence likely varied more than if the trial were more prescriptive. Another limitation was that 36.2% of the sample were men, 24.5% were not White, 23.4% were not college graduates, and the sample was somewhat affluent, which restricts our ability to generalize the results. In addition, 15 of the participants were in the trial when the COVID-19 pandemic began in the United States in 2020, which might have affected their results and decreased our sample size.
We had recruitment challenges, in part, because participants had to have a measured, laboratory-based BP ≥130 mm Hg despite any BP medications. Of the 138 people who were eligible based on initial screening, 95 (69%) were eligible after baseline BP measurement. This suggests that requiring an in-laboratory elevated baseline BP to confirm hypertension might have been too stringent.
In the MHERO trial of an online 4-month intervention, compared with a standard-of-care DASH dietary pattern, a VLC dietary pattern showed improvements in BP, glycemic control, and weight in adults with hypertension, prediabetes or type 2 diabetes, and overweight or obesity. These results provide initial evidence that a VLC dietary pattern might be more appropriate than the DASH dietary pattern for short-term disease management for these high-risk adults, and thus might have implications for clinical practice guidelines. Future research with larger samples, longer follow-up periods, and long-term outcomes is warranted.
Acknowledgments
We thank our dedicated participants and our team, which included Rosanna Spicer, Caitlyn Hamilton, Gabrielle Blackshire, Kathleen Lopez, Justin Flood, Franco Szeto, Paige Carrigan, Mario Pallazola, Deanna J. Marriott, and Bradley Liestenfeltz. Thank you to consultants Ronald M. Krauss, Paul R. Conlin, Heather M. Johnson, and Sarah Kim. Thank you to the Michigan Nutrition Obesity Research Center for assistance in collecting 24-hour dietary recalls, and to the University of Michigan Consulting for Statistics, Computing, and Analytics Research and the Michigan Institute for Clinical and Health Research for statistical advice.
Footnotes
Conflicts of interest: L.R.S.’s partner, H.B., is an inventor of software used in this study, which purchased a software services agreement for its use.
Funding support: This research was supported by an Early Implementation Grant from the University of Michigan Interprofessional Exchange Research Stimulus program led by the deans of the Health Sciences Council at the University of Michigan, for which L.R.S. was the principal investigator, and L.M.J., J.A.W., H.L.D., and C.R. were coinvestigators. L.R.S. was also supported by a K01 award from the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (DK107456). L.R.S. received funding from Sentinel Management, the Milken Foundation, and the Baszucki Brain Research fund to support research on the effect of a ketogenic diet for severe mental illness. L.M.J. was also supported by a K01 award from the NIH National Heart, Lung, and Blood Institute (HL145366) and a fellowship from the Gordon and Betty Moore Foundation (GMBF9048). J.A.W. was supported by the NIH NIDDK (DK119166). A.L.M was supported as a Robert Wood Johnson Foundation Future of Nursing Scholar. The blood tests were supported in part by a grant to the Michigan Diabetes Research Center from the NIDDK (P30DK020572). The dietary recalls used Core Services supported by a grant to the University of Michigan from the NIH (DK089503). The funders had no role in data collection and analysis, decision to publish, or preparation of the manuscript.
Trial registration number: NCT03729479
- Received for publication June 28, 2022.
- Revision received January 14, 2023.
- Accepted for publication January 24, 2023.
- © 2023 Annals of Family Medicine, Inc.