Abstract
PURPOSE The impact of digital health on medically underserved patients is unclear. This study aimed to determine the early impact of a digital innovation to grow quality care through an interprofessional care team (DIG IT) on the blood pressure (BP) and 10-year atherosclerotic cardiovascular disease (ASCVD) risk score of medically underserved patients.
METHODS This was a 3-month, prospective intervention study that included patients aged 40 years or more with BP of 140/90 mmHg or higher who received care from DIG IT from August through December 2021. Sociodemographic and clinical outcomes of DIG IT were compared with historical controls (controls) whose data were randomly extracted by the University of California Data Warehouse and matched 1:1 based on age, ethnicity, and baseline BP of the DIG IT arm. Multiple linear regression was performed to adjust for potential confounding factors.
RESULTS A total of 140 patients (70 DIG IT, 70 controls) were included. Both arms were similar with an average age (SD) of 62.8 (9.7) years. The population was dominated by Latinx (79.3%) persons, with baseline mean BP of 163/81 mmHg, and mean ASCVD risk score of 23.9%. The mean (SD) reduction in systolic BP at 3 months in the DIG IT arm was twice that of the controls (30.8 [17.3] mmHg vs 15.2 [21.2] mmHg; P <.001). The mean (SD) ASCVD risk score reduction in the DIG IT arm was also twice that of the controls (6.4% [7.4%] vs 3.1% [5.1%]; P = .003).
CONCLUSIONS The DIG IT was more effective than controls (receiving usual care). Twofold improvement in the BP readings and ASCVD scores in medically underserved patients were achieved with DIG IT.
INTRODUCTION
Medically underserved populations are 40% more likely to have hypertension and 3 times more likely to die from heart diseases due to uncontrolled high blood pressure (BP).1,2 The COVID-19 pandemic further contributed to the increased morbidity and mortality among people with uncontrolled chronic diseases due to compromised immunity and interrupted access to care. Over the course of the pandemic, the mortality rate of medically underserved patients with chronic diseases was twice that of the general public with chronic conditions.3
Digital health technologies such as self-monitoring devices and apps are becoming increasingly important as tools to promote healthy habits, medication adherence, and patient empowerment. In recent years, studies4,5 have showcased the positive impact of mobile and web-based platforms on patients with and without chronic diseases. In addition, advancements in technology have equipped both health care teams and patients with the flexibility to communicate without routine trips into the office, bypassed disruptions to timely access to care, improved appointment no-show rates, and overcome day-to-day operational challenges such as limited space or shortage of medical staff.6
While numerous studies have examined the effectiveness of health digital devices on patient outcomes, the findings are largely mixed, have different study designs, and minimal inclusion of medically underserved patients. In a meta-analysis7 that evaluated 15 randomized controlled trials with a total of 7,415 patients with hypertension, the mean change in the systolic blood pressure (SBP) between the app-based intervention and usual care ranged from −7.0 mmHg to +5.7 mmHg. These studies did not intentionally include medically underserved patients; hence their findings may not be generalizable to patient populations who are economically and socially disadvantaged or have limited health access or resources. Similarly, in another meta-analysis8 that included 32 studies with a total of 11,395 patients, BP control was 23.7% (risk ratio = 1.226, P <.001) higher among patients who received remote BP monitoring. The between-study heterogeneity was substantial (I2 = 70.656%; P <.001), with less than 36% of the studies declaring inclusion of patients from medically underserved areas. The impact of remote BP monitoring on medically underserved patients, therefore, is inconclusive at this time.
During the height of the COVID-19 pandemic, we developed and implemented a comprehensive medication management service in a Federally Qualified Health Center (FQHC) to manage patients with chronic conditions such as uncontrolled hypertension. We incorporated digital innovation to grow quality care through an interprofessional care team (DIG IT) in medically underserved areas of Orange County, California. Specifically, DIG IT embraced remote patient monitoring of BP that interfaced with the electronic health records (EHRs), allowing clinical pharmacists to monitor patients remotely and adjust medications via telemedicine. The purpose of DIG IT was to achieve effective and timely BP control in a safe environment to ensure equitable access and quality care for the management of hypertension. The objectives of this study were to determine the impact of DIG IT on the BP and atherosclerotic cardiovascular disease (ASCVD) risk scores of medically underserved patients.
METHODS
Study Design and Settings
This was a prospective, nonrandomized intervention study designed to compare patients who received DIG IT for BP management vs those who did not. Those who did not receive DIG IT received usual care, also known as historical controls (controls). The study was conducted in a university-affiliated FQHC located in 2 cities (Santa Ana and Anaheim) in Orange County, California. The FQHC is part of an academic health system, providing primary medical care to approximately 27,000 medically underserved patients who are predominantly Latinx and monolingual Spanish speakers. More than 95% of these patients live below 200% of the federal poverty level, and 11% are uninsured. This study was approved by the University of California, Irvine’s Institutional Review Board (UCI IRB #1558).
Inclusion and Exclusion
In the DIG IT arm, all patients aged 40 years or more with a formal diagnosis of essential hypertension and uncontrolled high BP (defined as more than 140/90 mmHg)9 from August through December 2021 were included in this study. Patients in the DIG IT arm with less than 3 months of BP readings or missing data at baseline and at 3 months were excluded from data analysis. The same inclusion and exclusion criteria were applied to the controls that received usual care for BP management.
DIG IT Blood Pressure Management (Intervention)
DIG IT combines digital health monitoring technology, comprehensive medication management, and patient-centered care through interprofessional collaboration to address uncontrolled hypertension among medically underserved populations. Established in October 2020, DIG IT consisted of family physicians, nurse practitioners, physician assistants, and clinical pharmacists who are supported by an information technology (IT) team comprised of a physician informaticist, registered nurses, and medical assistants. Eligible patients were identified by their clinicians and coached by the IT team on the use of the BP digital devices loaned to them free of charge. The BP digital devices allowed self-monitoring of BP readings to be recorded and interfaced with the clinic’s EHR system, making it possible for health care team members to view patients’ home BP readings real-time and receive alerts about high BP readings via EHR. Clinical pharmacists tracked patient’s BP readings remotely and conducted telemedicine interviews with the patients every 2 to 4 weeks, depending on BP control and patient preference, and actively adjusted BP medications under a collaborative practice agreement. In addition, the DIG IT and the IT teams work in the same open clinic space to facilitate effective communication. To maintain the sustainability of the DIG IT, patients who achieved BP control were discharged from the DIG IT program and the digital device was refurbished and passed on to assist the next eligible patients with uncontrolled hypertension.
Usual Care Blood Pressure Management (Control)
We leveraged the University of California Health Data Warehouse, a comprehensive, deidentified repository of EHR and claims data from the University of California Irvine (UCI) Health Medical Center. Upon IRB approval, the principal investigator of the study placed the request for data acquisition to informatics officers from the UCI Health Enterprise Data and Analytics. Matched controls (1:1, based on age, ethnicity, and baseline BP readings) were identified and randomly extracted from the UCI Health Enterprise Data Warehouse during the same time period that the DIG IT (intervention) data were collected. The deidentified control data were then made available to the principal investigator and the study team for analysis via a secured portal. Matched variables (non-modifiable risk factors) were selected based on their documented effects on BP outcomes as delineated in the 2017 American College of Cardiology and American Heart Association Clinical Guidelines for High Blood Pressure.10 In addition, data from FQHC were excluded to ensure no overlap of data between the 2 groups.
Patients from the control group received BP management from clinicians through in-person clinic visits without the involvement of digital devices, telecommunication technologies, or contact with pharmacists except when collecting medications from a pharmacy. Blood pressure readings were collected at baseline, at 2 months, and at 3 months. An addition of 1-month window after the 3-month time period was allowed to account for the general BP monitoring frequency for control to reflect the usual care.
Outcomes
Outpatient BP readings were followed and extracted at baseline and monthly for a total of 3 months for the DIG IT and control arms. An average of daily BP readings was used when there were multiple measurements within the same visit, day, or month. The 10-year ASCVD risk scores were calculated at baseline and at 3 months for both arms using the ASCVD Risk Estimator from the American College of Cardiology using the health and lifestyle factors such as diabetes status, lipid profile, BP, and smoking statutes as appropriate.
Analysis
Descriptive analysis (mean, SD) was utilized to describe the demographic data and clinical outcomes. Chi-square test, Fisher’s exact test, and Student’s t-test were utilized to compare the demographic and clinical characteristics between the 2 groups. Multiple logistic and multiple linear regressions were performed to adjust for potential confounding factors (gender, lipid profile, self-reported alcohol consumption status) associated with the study outcomes. Statistical analysis was performed using the IBM SPSS Statistics 22 (IBM Corp) program.
RESULTS
Demographics
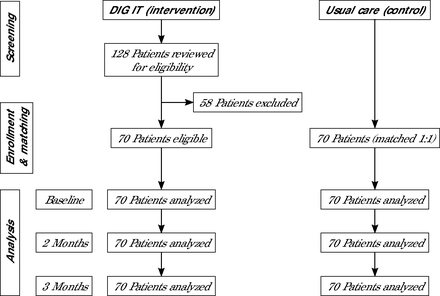
From August through December 2021, 128 patients were referred to DIG IT. Of these, 58 (45.3%) were excluded due to loss of contact after verbally agreeing to the enrollment, missing the first appointment to receive remote BP device, experiencing homelessness, or losing access to Wi-Fi required for remote monitoring (Figure 1). This resulted in 70 (54.7%) patients included for final analysis for the DIG IT arm and 70 matched controls for a total of 140 participants (Table 1).
Flow of participants (N = 140).
DIG IT = digital innovation to grow quality care through an interprofessional care team; Wi-Fi = wireless network for internet access.
Baseline Patient Demographics and Clinical Parameters (N = 140)
The sociodemographic data between the 2 arms were similar with an average age (SD) of 62.8 (9.7) years, and dominated by Latinx (79.3%) people. The self-reported smokers (2.9%), prevalence of diabetes (53.6%), baseline mean BP (163/81 mmHg), and baseline 10-year ASCVD risk scores of 23.9% among the 2 arms were also similar. Compared with control group, the DIG IT arm had more males (70.0% vs 51.4%; P <.01) with higher mean (SD) total cholesterol level (179.4 [36.6] mg/dL vs 153.1 [39.6] mg/dL; P <.001), and a lower proportion of self-reported alcohol consumption (18.6% vs 47.1%; P <.001).
Outcomes: Blood Pressure
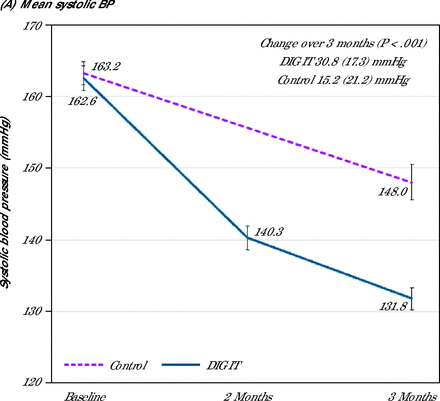
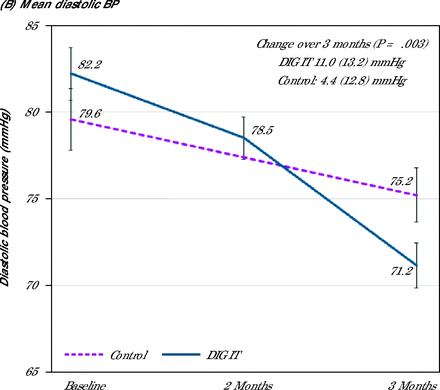
Both mean (SD) SBP (131.8 [13.0] mmHg vs 148.0 [20.4] mmHg; P <.01) and diastolic blood pressure (DBP) (71.2 [10.8] mmHg vs 75.2 [13.2] mm Hg; P = .049) at 3 months were lower in the intervention arm compared with the control arm. After adjusting for clinically important factors and baseline BP, both SBP (P <.001) and DBP (P = .029) at 3 months remained statistically significant (Supplemental Table 1). The mean change in the SBP and DBP from baseline to 3 months was significantly greater in the DIG IT arm. The mean SBP change for DIG IT was from 163 mmHg at baseline to 132 mmHg at 3 months, and the mean SBP change for control was from 163 mmHg at baseline to 148 mmHg at 3 months (P <.001). The mean DBP change for DIG IT was from 82 mmHg at baseline to 71 mmHg at 3 months, and mean DBP change for control was from 80 mmHg at baseline to 75 mmHg at 3 months (P <.049) (Figure 2). Reduction in SBP and DBP over the 3-month period in the DIG IT arm was double that of the control group. Mean (SD) reduction of SBP DIG IT was 30.8 (17.3) mmHg vs control at 15.2 (21.2) mmHg (P <.001), and DBP DIG IT was 11.0 (13.2) mmHg vs control at 4.4 (12.8) mmHg (P = .003).
Change in (A) mean systolic blood pressure, and (B) mean diastolic blood pressure over 3 months.
BP = blood pressure; DIG IT = digital innovation to grow quality care through an interprofessional care team.
Note: Change over time data presented as mean (SD) with P values.
While trends of consistent BP improvement were observed monthly in the DIG IT arm with a mean SBP of 140 mmHg at 2 months, this observation was not available for patients in the control as patients in the control were only followed up minimally at 3 months, which reflected the general usual care practice. Overall, 51 (72.9%) patients in the DIG IT arm and 25 (37.1%) patients in the control achieved the BP target of less than 140/90 mmHg within 3 months. After adjusting for gender, lipid profiles, and self-reported alcohol consumption status, patients in the DIG IT arm were 5.10 (95% CI, 2.13-12.0; P <.001) times more likely to achieve BP target compared to the control (Supplemental Table 2).
Outcomes: ASCVD Risk Score
Mean (SD) ASCVD scores at 3 months (17.4% [15.2%] vs 20.9% [17.2%]; P <.204) were similar when compared between the intervention and the control. The ASCVD score at 3 months, however, was statistically significant (P = .001) after adjusting for clinically important factors and baseline ASCVD score (Supplemental Table 1). The mean (SD) baseline to 3-month 10-year ASCVD risk scores for the DIG IT arm and control were 23.8% (19.4%) to 17.4% (15.2%) and 24.0% (18.3%) to 20.9% (17.2%), respectively. The mean (SD)10-year ASCVD risk score reduction in the DIG IT arm was twice that of the control (DIG IT 6.4% ([7.4%]; control 3.1% [5.1%]; P = .003). The difference in the mean change of 10-year ASCVD risk score remained statistically significant (P = .002) after adjustment of confounding variables including gender, lipid profile, and self-reported alcohol consumption status (Supplemental Table 3).
DISCUSSION
While studies have examined the impact of digital health among the general public living in urban or rural areas, medically underserved patients are often underrepresented. There are few studies that attempt to evaluate the effects of digital health in disadvantaged minority populations. This is one of the first studies that intentionally evaluated the impact of digital technologies on the clinical outcomes of medically underserved, high-risk patients with uncontrolled hypertension in a FQHC. In this study, we found that compared with controls, patients who received DIG IT achieved twice as much improvement in their BP readings and 10-year ASCVD risk scores within 3 months. The number of patients meeting the BP target within 3 months in the DIG IT arm was also double that of the control group.
In this study, mean SBP in the DIG IT arm was lowered by 31 mmHg vs 15 mmHg in the controls over the 3-month period. Monthly observation of BP change in the DIG IT group further revealed that SBP improvement over 1 month was more than what the control was able to achieve over the 3-month period. Large epidemiological studies have shown that lowering SBP by 10 mmHg or DBP by 5 mmHg across all levels of BP, not only in hypertension, reduced the risk of major cardiovascular disease events by 20%, stroke by 27%, and deaths from all causes by 13%.11,12 While our study did not evaluate actual cardiovascular incidents, the reduction in the 10-year ASCVD risk scores, which were twice that of the controls, suggested substantial benefits of cardiovascular risk reduction achieved through DIG IT.
As confirmed in numerous studies,13,14 timely BP improvement and target attainment as observed in our study were most likely due to the shorter encounter intervals provided by our care team. In a retrospective cohort study of 5,042 patients with hypertension that examined the relationship between encounter frequency and BP target attainment, a 1 month increase in the average encounter interval was significantly associated with a hazard ratio of 0.764 for BP normalization.16 In addition, BP readings measured by the DIG IT patients from the comfort of their home or in a familiar environment may have also contributed to the overall BP improvement by removing visit-to-visit BP variability due to factors such as white coat syndrome that are commonly observed in ambulatory care settings.10,16 With the use of digital technology, clinical pharmacists in our study were able to reach out to the DIG IT patients in a timely manner based on patient-measured BP readings, minimizing patient no-show rates and bypassing long wait times for an in-person visit.
Due to the complex interplay among study investigator biases and individual and community factors, medically underserved patients are historically underrepresented in clinical trials. This limits the generalizability of study findings to at-risk populations with social determinants of health.17,18 Specifically, low e-literacy and education level have been highlighted as barriers to successful digital health adoption among the medically underserved.19 Furthermore, lack of data interface between a patient’s own self-measured readings and the EHR is a weakness in the infrastructure of digital health that hinders timely care and treatment awareness. In our study, the digital devices and the EHR were interfaced, allowing all health care team members within the health system the access to patient’s self-measured readings. In addition, an IT team consisting of registered nurses, medical assistants, and a physician informaticist were available to address any technology issues related to the use of the digital devices. All patients were coached by the IT team on the use of the digital device before embarking on DIG IT. The IT team was readily available to support the DIG IT care team through troubleshooting, allowing clinicians to focus on patient care. This effective digital health delivery, empowered by our IT team, also contributed positively to our study findings.
In our study, the incorporation of interprofessional care team models aided patients in reaching BP goals. Numerous studies have affirmed that collaboration among pharmacists, clincians, and other clinical team members helps to reduce hospitalization rates and costs.20,21 In a randomized controlled trial of 411 patients, a pharmacist-involved collaborative care model was found to achieve better clinical outcomes, patient satisfaction, and cost reduction compared with usual care.21 In our study, clinical pharmacists provided patients with medication optimization through comprehensive medication management under a collaborative practice agreement. This allowed primary care clinicians additional time to attend to complicated cases that require in-depth assessments. Tailoring patient-centered care around patients’ needs fosters not only interprofessional collaboration, but also shared decision-making between patients and clinicians to achieve positive outcomes.
This study was not without limitations. First, patients from control, though from the same university health system, were not directly recruited from the patient cohort in our FQHC. This was to minimize cross-contamination of interventions provided by our team of interprofessional healthcare clinicians at FQHC. Although every effort was made to ensure similarity between the 2 groups by matching the study period, baseline demographics of the patients in DIG IT, and confounding factors, the inherent limitation of using a historical control group may have introduced unfair comparisons of both known and unknown outcome predictors and affected the actual effect size. Also, we only observed the first impact or early effect of DIG IT over 3 months. While our study established the timely and effective results of DIG IT, further study is needed to examine the sustainability of the program. Finally, we did not evaluate humanistic outcomes such as digital health-related satisfaction rate, distress level due to technological barriers, demand, and economic outcomes which include direct and indirect costs. With positive clinical outcomes established, future studies may examine the sustainability of clinical outcomes and the impact of digital health on the humanistic and economic outcomes of medically underserved patients. Furthermore, future work should include comparisons among multiple nonprofit health centers dedicated to underserved communities to minimize heterogeneity and maximize similarities among the medically underserved patients.
CONCLUSION
Compared with the control group, collaborative remote patient monitoring of medically underserved patients with uncontrolled hypertension through DIG IT appeared to be more effective than usual care as it achieved twofold improvements in BP readings and ASCVD risk scores. The unique feature of DIG IT, which included an interprofessional approach to provide remote patient monitoring interfaced with EHR, likely contributed to the positive outcomes achieved by these medically underserved patients.
Acknowledgments
The authors would like to acknowledge Dr Jeffrey Arroyo for his clinical and technical guidance during the initial set up of DIG IT; Julia Torres, RN and her team at UCI Health Family Health Center for providing technical and patient care support; and the FQHC’s outreach care team, Senior Manager/Assistant Operations Director Leanne Funada, and Practice Manager Juan C. Macedonio, at UCI Health Family Health Center for their administrative support.
Footnotes
Conflicts of interest: authors report none.
Previous presentations: A version of this report was presented as a poster at the California Society of Health-System Pharmacists Seminar; October 12-15, 2023; Long Beach, California; and also at the 2022 American College of Clinical Pharmacy Global Conference on Clinical Pharmacy; October 15-18, 2022; San Francisco, California.
- Received for publication December 21, 2023.
- Revision received June 13, 2024.
- Accepted for publication June 13, 2024.
- © 2024 Annals of Family Medicine, Inc.