Abstract
PURPOSE For most guidelines, diagnosis of community-acquired pneumonia (CAP) is based on a combination of clinical signs and focal consolidation visible on chest radiographs (CRs). Our objective was to analyze antibiotic initiation by general practitioners for patients with suspected CAP according to CR.
METHODS We conducted a prospective cross-sectional study in general practice in France. From November 2017 to December 2019, adult patients with clinically suspected CAP after CR were included. Radiographs were categorized as CAP positive or CAP negative. We analyzed patient characteristics and antibiotic initiation according to CR results.
RESULTS A total of 259 patients were included in the study. Median age was 58.0 years (interquartile range, 41.0-71.0 years); 249 (96.1%) patients had not received antibiotics before inclusion, and 144 (55.6%) had a positive CR. Patients with positive CR were clinically more severe than those with negative CR, with longer-lasting symptoms. Antibiotics were initiated for 142/143 (99.3% [95% CI, 97.9%-100.0%]) patients with positive CR and 79/115 (68.7% [95% CI, 60.2%-77.2%]) with negative CR (P < .001). Among the 115 CR-negative patients, clinical characteristics that were significantly different between patients for whom antibiotics were initiated or not did not appear to be clinically relevant.
CONCLUSIONS For patients with suspected CAP, general practitioners systematically took into account results of positive CRs to initiate antibiotics and took much less account of negative CRs. These results justify clarification of what should be done in cases of clinical suspicion of CAP without radiologic confirmation.
INTRODUCTION
Community-acquired pneumonia (CAP) is a common and potentially severe disease. In 2016, lower respiratory tract infections were reported to be responsible for 2.4 million deaths worldwide.1 Diagnosis of CAP is complex and based on a combination of nonspecific clinical symptoms and radiologic criteria. According to most guidelines, confirmation by chest radiography (CR) is needed to establish a diagnosis of CAP and initiate antibiotic therapy, so as to limit antibiotic prescriptions to pneumonia and not to other lower respiratory tract infections. Making the administration of antibiotics conditional on the presence of radiologic signs of CAP should decrease antibiotic consumption, which is a major strategy for preventing antimicrobial resistance, a major global threat.2,3 However, there are some discrepancies among guidelines on the role of CR.4-9 According to French guidelines, CAP diagnosis must be established via visualization of parenchymal opacity on CR.4,5 In the United States, CR is also necessary to confirm CAP diagnosis, although it is recognized that CAP is commonly diagnosed without the use of CR, especially in the ambulatory setting.8 In the United Kingdom, CR is not mandatory for suspected CAP unless the diagnosis is doubtful, the evolution under treatment is not favorable at review, or in the case of suspected underlying lung pathology.6,7 In the European guidelines, a diagnosis of CAP is definite if CR shows lung shadowing that is likely to be new. However, in the European guidelines for outpatients, CR should only be considered to establish a CAP diagnosis in the case of persisting doubt after C-reactive protein (CRP) testing.9 Indeed, some countries, such as the Netherlands, recommend the use of point-of-care tests (eg, for CRP) in their CAP diagnostic strategy.10
Implementing CR in the case of clinical CAP suspicion must take into account CR cost and the cost of equipment needed to make CR accessible. These costs should be weighed against the benefit of the potential decrease in antibiotic consumption. Beyond the presumed necessity of CR and the possibility in daily practice of performing CR without delay, it is important to analyze antibiotic initiation by general practitioners (GPs) for suspected CAP according to CR results.
In one study involving the clinical scenario of suspected CAP, a positive CR supported the diagnosis of CAP for almost all practitioners.11 However, regarding the same clinical scenario with a negative CR, the median estimated probability of pneumonia by practitioners was 50% (interquartile range [IQR], 30%-80%), and 401 (72.5%) practitioners would treat these patients with antibiotics.11 In a prospective cohort study conducted in Switzerland, 48.3% of patients without radiologic signs of CAP received antibiotics.12 In contrast, in another interventional study conducted in Sweden, this rate was 24%.13 These discordant results do not allow for a definition of the influence of CR in the management of patients with a clinical suspicion of CAP.
Given that French recommendations do not sufficiently specify the course of action in terms of initiating antibiotics in cases of clinical suspicion of CAP with a negative CR,4,5 we determined that it might be important to generate additional data evaluating the effect of CR results on GPs’ decisions to initiate antibiotics for patients with suspected CAP. We therefore performed a prospective primary care study in France in which CR was mandatory within 6 hours of CAP suspicion. We describe the clinical characteristics and management of patients with suspected CAP according to CR results.
METHODS
Study Design
We conducted a prospective cross-sectional study from November 1, 2017 to December 31, 2019 in metropolitan France. We worked with a network of 277 GPs who specifically enrolled adult patients with suspected CAP and excluded patients with influenza-like illness or bronchitis. A reminder of the current French CAP guidelines was provided at each enrollment.
We defined clinical suspicion of CAP as the presence of ≥ 1 general symptom of infection (fever > 38.5 °C, heart rate > 100 beats per minute (bpm), respiratory rate > 20 breaths per minute, global impression of severity, fatigue, chills) and ≥ 1 symptom or clinical sign of pulmonary localization (cough, unilateral chest pain, sputum, focus of crackles). For each patient, CR was performed as standard of care according to French guidelines. For patients to be included, a CR performed within 6 hours after the initial visit was mandatory except for those requiring immediate hospitalization (Supplemental Figure 1). Each CR was categorized by a local radiologist as suggestive of CAP (CR+) or not (CR−).
Each GP determined whether to initiate antibiotic therapy on the basis of the interview, clinical examination, and CR interpretation. Patients were contacted by the investigating GP by telephone on day 28 to collect follow-up information regarding symptom duration, follow-up visits, and hospitalization.
We collected data with an electronic case report form from the day of inclusion to day 28. Patient characteristics, conditions increasing the risk of invasive pneumococcal disease, immunization status,14 symptoms at inclusion, clinical examination data, CR results, number of days leave from work/school activities prescribed, and prescribed treatment were reported at inclusion (day 0).
Objectives
The main objective was to describe antibiotic initiation by GPs according to CR results. The secondary objective was to identify factors associated with antibiotic initiation.
Statistical Analysis
We described patient characteristics using No. (%) for categorical variables and median or IQR for continuous variables. To analyze factors associated with antibiotic initiation, we compared patient characteristics at inclusion and at 28 days between CR+ and CR− patients. For CR− patients, we also compared characteristics according to antibiotic initiation (yes/no) by GPs. We used the Wilcoxon test for comparisons of continuous variables and the Fisher exact test for comparisons of categorical variables.
Ethics
This study is ancillary to the PneumoCAP study, which was sponsored by the French National Academic Council of General Practice (Collège National des Généralistes Enseignants Conseil) and funded by Pfizer Inc. The French health authority (National Agency for the Safety of Medicines and Health Products [ANSM]) and the Institutional Review Board for the Protection of Human Subjects approved the study protocol and patient informed consent procedures. All enrolled patients provided written informed consent for inclusion. The protocol was registered on the clinicaltrials.gov website under the PneumoCAP acronym (NCT03322670). The French Ethics Committee (Comité de Protection des Personnes) approved the study protocol.
RESULTS
Population Description
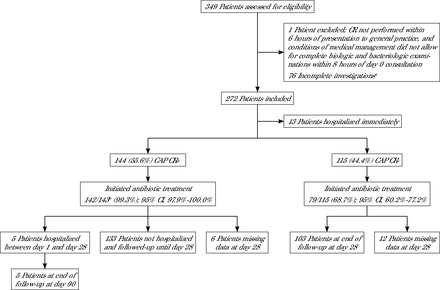
A total of 272 patients with suspected CAP were included by 108 GPs. Among the 272 patients, 13 were immediately referred to a hospital emergency department without CR, which led to a final study population of 259 patients (Figure 1). The median age was 58.0 years (IQR 41.0-71.0 years), 139 (53.7%) were female, 249 (96.1%) had not received antibiotics before inclusion, and 69 (26.7%; 1 missing value) had ≥ 1 risk factor for invasive pneumococcal disease (risk factors for invasive pneumococcal infection were defined according to the French vaccine schedule 2017: chronic respiratory disease, asthma, chronic heart failure, nephrotic syndrome, asplenia or splenectomy, chronic liver disease, homozygous sickle cell disease, HIV infection, immunocompromising conditions, diabetes, cerebrospinal fluid leak, cochlear implant) (Table 1).14 The 3 most common symptoms at inclusion were cough in 251 patients (96.9%), tiredness in 244 (94.2%), and fever in 180 (70.0%; 2 missing values). Crackles were noted in 166 patients (64.1%) and ronchi in 83 (32.0%). Among the 259 patients, 144 (55.6%) had a positive CR, and 115 (44.4%) had a negative CR.
Flowchart and antibiotic prescription.
CAP = community-acquired pneumonia; CR = chest radiography.
aIncomplete investigations = patients with suspected CAP who could not have CR within 6 hours either due to unavailability of the radiology office or because they were unable to go to the radiology office.
bData missing for 1 patient (n = 143).
Comparison of CAP CR+ and CAP CR−: Patient Characteristics, Conditions With Increased Risk of Invasive Pneumococcal Disease, History of Pneumonia, Vaccination Status, Symptoms on Inclusion and Clinical Examination, Follow-Up, and Data on Day 28
Investigator Descriptions
Investigators were mostly female (153/277 [55.2%]), with a median age of 39.0 years (IQR 32.5-53.5 years), and 108 (39%) included ≥1 patient; 210 (76.1%) were GP trainers (GPs who supervise medical students), practicing in the whole of metropolitan France (168 [62%] in urban areas).
Patient Characteristics and Management by GPs According to CR Results
Regarding patient comorbidities, there was no difference between CR+ patients and CR− patients except for diabetes, which was more common in CR+ patients (11.2% vs 2.6%; P = .008) (Table 1). Patients who were CR+ reported more dyspnea (53.5% vs 40.0%; P = .034) and unilateral chest pain (36.8% vs 21.7%; P = .010); they also had higher body temperature (37.7 °C [IQR 37.0-38.3 °C] vs 37.3 °C [IQR 37.0-38.0 °C]; P = .008), faster heart rate (90.0 bpm [IQR 80.0-102.0 bpm] vs 82.0 bpm [IQR 71.0-95.0 bpm]; P = .002) and faster respiratory rate (18.0 breaths/min [IQR 16.0-22.0 breaths/min] vs 16.0 breaths/min [IQR 15.0-20.0 breaths/min]; P < .001) compared to CR− patients.
Physical examination of CR+ patients more commonly revealed decreased breath sounds (33.3% vs 20.0%; P = .018) compared to CR− patients; however, they less commonly exhibited wheezing (11.8% vs 21.7%; P = .041) (Table 1). Regarding follow-up data, CR+ patients had a longer fever duration (3.0 days [IQR 1.0-5.0 days] vs 2.0 days [IQR 0-4.0 days]; P = .001), longer dyspnea duration (4.0 days [IQR 0-12.0 days] vs 0 days [IQR 0-10.0 days]; P = .035), and reported more follow-up visits (38.9% vs 0%; P < .001) than CR− patients. Antibiotics were initiated for 142/143 (99.3% [95% CI, 97.9%-100.0%]) CR+ patients (no antibiotic information for 1 patient) and for 79/115 (68.7% [95% CI, 60.2%-77.2%]) CR− patients (P < .001) (Figure 1).
Differences Between CR− Patient Characteristics According to Antibiotic Initiation
Table 2 lists characteristics of CR− patients according to whether antibiotics were initiated by the GP during the initial visit. Among the 115 CR− patients, there were no differences in comorbidities, duration of symptoms preceding the day of inclusion, impression of severity, and auscultatory abnormalities between patients for whom antibiotics were initiated and those who were not. Those for whom antibiotics were initiated had greater incidences of tiredness (98.7% vs 86.1%; P = .011) and ear, nose, and throat symptoms (63.3% vs 38.9%; P = .017), faster heart rate (86.0 bpm [IQR 72.0-100.0 bpm] vs 80.0 bpm [IQR 70.0-88.0 bpm]; P = .024) and faster respiratory rate (17.0 breaths/min [IQR 15.0-20.0 breaths/min] vs 15.0 breaths/min [IQR 14.0-17.5 breaths/min]; P = .001); however, oxygen saturation tended to be lower (97.0% [IQR 94.5%-98.0%] vs 97.0% [IQR 96.0%-98.0%]; P = .037). Regarding follow-up data, CR− patients with antibiotic initiation had longer dyspnea duration (3.0 days [IQR 0-10.0 days] vs 0 days [IQR 0-6.0 days]; P = .038) than patients without antibiotics; all patients were alive in both groups at day 28 whether or not antibiotics were initiated at inclusion.
Comparison of CAP CR− With Antibiotic and CAP CR− Without Antibiotic: Patient Characteristics, Conditions With Increased Risk of Invasive Pneumococcal Disease, History of Pneumonia, Vaccination Status, Symptoms on Inclusion and Clinical Examination, Follow-Up, and Data on Day 28
DISCUSSION
In this study, in which systematic CR was an integral part of the management of suspected-CAP, we were able to specifically explore how GPs took into account CR results in their decision to initiate antibiotics. The initiation of antibiotics was almost systematic (99.3%) for CR+ patients and was very common (68.7%) for CR− patients. Considering the French recommendations for the management of pneumonia, we would have expected less antibiotic initiation for CR− patients; even more so, these patients appeared clinically less severe at inclusion than CR+ patients. Similarly, among CR− patients, the differences in clinical symptoms between those who did and did not receive antibiotics did not appear to be sufficiently clinically relevant to justify prescription. It appears that GPs almost systematically took into account the results of positive CR to initiate antibiotics but took much less account of CR when it was negative, refuting a CAP diagnosis. Indeed, more than two-thirds of GPs initiated antibiotics despite a negative CR. In this population of younger patients with few comorbidities managed by GP trainers, we would have expected strict adherence to CAP guidelines and a greater difference in the incidence of antibiotic initiation between CR+ patients and CR− patients.
Whereas the CAP diagnostic certainty of CR− patients is questionable, we can also question the reasons that led GPs to initiate antibiotics for these patients. One explanation could be that GPs considered the absence of opacity on CR to be the consequence of radiologic delay. However, the time to symptom onset before inclusion and CR was not statistically different between CR+ and CR− patients (4.0 days [IQR 2.0-8.0 days] vs 4.0 days [IQR 2.0-7.0 days]; P = .280). Another explanation might be related to a low sensitivity of CR to diagnose CAP. In the Early CT-Scan for Community-Acquired Pneumonia at the Emergency Department (ESCAPED) study, one-third of CR− patients had CAP as revealed by chest computed tomography.15 A third explanation could be disagreement between GP and radiologist on CR interpretation, given that high interobserver variability in CR interpretation has been reported.16,17 Fourth, GPs who are not used to having ready access to CR in their routine practice might have become accustomed to making the decision to initiate antibiotics based on clinical data alone. Fifth, we can also believe that GPs initiated antibiotics for CR− patients because they retained another diagnosis (eg, pharyngitis, sinusitis) that requires antibiotics; when we excluded patients with ear, nose, and throat symptoms among CR− patients, 56.8% of patients still received antibiotics. Finally, our results could be explained by a premature closure bias in the clinical reasoning of GPs. Premature closure bias is the tendency to stop considering other possibilities after reaching a diagnosis.18
Strengths and Limitations
This study focused on patients with suspected CAP managed in primary care by GPs and for whom CR was systematically performed, which might have led to selection bias. Mandatory CR according to guidelines allows for specific exploration of the relation between CR results and antibiotic initiation, avoiding the GP decision of whether or not to call for CR. The time interval for CR completion was 6 hours after initial consultation, which might have had an effect on CR results. This short time interval was justified by the need to avoid delaying patient management and antibiotic initiation if necessary. Population characteristics are those expected in primary care with few comorbidities. Of note, very few patients (10 [3.9%]) had received antibiotics before inclusion, which is ideal for studying clinical and radiologic presentation and decision making. A sensitivity analysis stratified by age (<75 and >75 years) showed no difference in antibiotic initiation.
Inclusion criteria used in this study to establish CAP suspicion were clinical criteria and might differ from those of other studies. Flateau et al reported a high heterogeneity of CAP inclusion criteria in randomized controlled trials, with > 42 different CAP inclusion criteria combinations and different performances of these criteria to include true CAP.19 In the present study, we used inclusion criteria with strong validity, identical to those of the ESCAPED study conducted in France (2011-2013) in emergency units, the positive predictive value of which was > 75% compared with a reference standard established by an adjudication committee at day 28 based on CR and chest computed tomography results.15
Regarding interpretation of CRs by local radiologists, heterogeneity in the interpretation16,17 might have been incorrectly classified as positive or negative. Regarding investigator characteristics, GPs were selected from a GP research network for their ability to enroll patients with suspected CAP and to perform CR within 6 hours after the initial visit. Investigators were comparable to overall French GPs in terms of sex ratio but were significantly younger.20 It has been shown that results obtained with practice-based research networks are relevant to those obtained with other practicing clinicians21; however, GP trainers have been identified to prescribe fewer antibiotics than other GPs.22
Comparison With Existing Literature
The percentage of CR+ patients in this study (> 50%) was comparable to that in other studies conducted in primary care or in emergency units with ambulatory patients.15,23,24 Our results on antibiotic initiation according to CR results is concordant with those of Morgan et al, who interviewed practitioners regarding their decisions to initiate antibiotics; 99.6% (551/553) would have initiated antibiotics for patients with suspected CAP and positive CR and 72.5% (401/553) for CR− negative patients.11
In the present study, antibiotic initiation was almost systematic (99.3%) for CR+ patients, consistent with French recommendations.4,5 Our results suggest that CR is used by GPs to confirm their clinical diagnosis of CAP and support them in their willingness to initiate antibiotics. The main antibiotics prescribed were in accordance with French guidelines.4,5 The clinical characteristics of fever, chest pain, increased heart rate, and increased respiratory rate associated with a positive CR in the present study have also been reported in the literature to be associated with positive CR or confirmed CAP.24-26
Implications for Research and Practice
It would be interesting to evaluate the clinical effect, in terms of morbidity and mortality, between initiating or not initiating antibiotics for patients with clinical suspicion of CAP and with negative CR. In the present study, there was no difference between CR− patients who received antibiotics and those who did not in terms of hospitalization during the 28-day follow-up period or death.
It would also be important to better define the predictive clinical signs of CAP. As described by Flateau et al, there is considerable heterogeneity in the inclusion criteria selected to define CAP in randomized controlled trials.19 In addition, exploratory qualitative research would be needed to understand the reasoning of GPs for antibiotic initiation for CAP. It would also be interesting to explore the extent to which biomarkers, such as point-of-care CRP, would allow GPs to have confidence in the negativity of CR.27
The process leading to antibiotic initiation for patients with suspected CAP is complex. Despite the absence of radiologic confirmation, most practitioners initiated antibiotics in the case of clinical suspicion of CAP. The effect of a negative CR on the antibiotic initiation decision appears to be low. This raises questions regarding the role of CR in the management strategy for CAP and justifies clarification of the guidelines as to what should be done in case of clinical suspicion of CAP without radiologic confirmation. In the present study, we found no clinically relevant difference between patients with clinically suspected CAP and negative CR who received antibiotics and those who did not. Nor was there any difference in the clinical outcome between these 2 groups of patients. Patients with a clinical suspicion of CAP (with no seriousness requiring hospitalization) and no radiologic confirmation should therefore be managed without antibiotics.
Acknowledgments
Clinical investigators: PneumoCAP study group; Scientific committee: Emmanuelle Varon, MD, and Sabine Trombert, MD; monitoring, data management, and statistical analysis: Christelle Auger, RN, URC-CIC Paris Centre.
Footnotes
Conflicts of interest: authors report none.
Funding support: This study is ancillary to the PneumoCAP study, which was sponsored by the Collège National des Généralistes Enseignants (CNGE). The PneumoCAP study was funded by Pfizer Vaccines and conducted as a research collaboration between CNGE as the sponsor and Pfizer Inc. The study funder had no role in data collection, study design, or data interpretation and analysis but contributed to reviewing this manuscript and the decision to publish.
Author contributions: Protocol conception and design: S.G., J.L.B., and H.P.; critical revision of the protocol: C.L., M.P.R., C.V., and X.D.; data acquisition: J.P., M.E., F.C., and R.P.; data analysis and interpretation: J.P., S.G., C.L., T.A., X.D., and J.L.B.; drafting of the manuscript: J.P., X.D., and J.L.B.; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: J.P., T.A., and C.L.; others who contributed significantly to this work are listed in the acknowledgments.
Ethical approval: Comité de Protection des Personnes, Ile de France II. Paris N° 2016-A01537 to 44.
- Received for publication September 5, 2023.
- Revision received June 18, 2024.
- Accepted for publication August 5, 2024.
- © 2024 Annals of Family Medicine, Inc.