Abstract
As the population ages, the prevalence of cognitive impairment due to neurodegenerative diseases such as Alzheimer disease (AD) is expected to double in the United States to nearly 14 million over the next 40 years. AD and related dementias (ADRD) are a leading cause of morbidity and mortality and among the costliest to society. Although emerging biomedical interventions for ADRD focus on early stages and are currently limited to AD, care management can benefit patients with ADRD across the disease course. Moreover, some causes of cognitive impairment are modifiable, and optimal overall management may slow or prevent additional decline. Nevertheless, a sizable proportion of cases of cognitive impairment among older adults remain undiagnosed. Primary care practitioners are often the first health care professionals to encounter cognitive concerns or to be able to observe changes in function resulting from cognitive impairment; hence, they have much to contribute to population health solutions for detecting cognitive impairment among older adults. In this report, we present key points and gaps in knowledge about methods for detecting cognitive impairment in primary care clinics. These were developed via an interdisciplinary Geriatrics Summit hosted by the National Academy of Neuropsychology in 2022, attended by representatives of national organizations engaged in work to improve care of older adults. We propose a novel workflow to facilitate detecting cognitive impairment during routine primary care, focusing on opportunities provided by the annual wellness visit, a preventive visit available to Medicare beneficiaries, along with additional recommendations and opportunities for clinical practice and research.
- cognitive screening
- cognitive impairment
- dementia
- Alzheimer’s disease
- neurodegenerative disease
- older adults
- elderly
- geriatrics
- family physicians
- primary care
- early detection
INTRODUCTION
As the global population continues to age, the prevalence of cognitive impairment impacting health and independence is expected to rise dramatically. For example, in the United States alone, the number of people living with Alzheimer disease (AD) is expected to double to nearly 14 million by 2060.1 Considerable efforts from diverse stakeholders are required to adequately prepare for the forthcoming surge in older adults seeking and requiring screening for cognitive impairment. Because most patients with dementia are managed in primary care,2,3 the National Academy of Neuropsychology hosted an interdisciplinary Geriatrics Summit in 2017 focused on identifying cognitive impairment in older adult patient populations within primary care settings, including both primary care clinics and emergency departments.4 One outcome of that Summit was formation of the Geriatric Emergency care Applied Research Network (GEAR; https://gearnetwork.org/about-gear-2/research-priorities-gear2/), which is funded by the National Institutes of Health to provide infrastructure to support collaborative, interdisciplinary research to advance dementia care in emergency departments.
In November 2022, the National Academy of Neuropsychology hosted a follow-up summit to engage in further discussion of low-resource, time-efficient methods for early detection of cognitive impairment in older adults in primary care clinics. The goals of the summit were to (1) review the current state of the science and practice in risk assessment and cognitive screening, including challenges to implementation, (2) arrive at a consensus on methods worthy of implementation and/or further validation, and (3) discuss future directions in research and clinical care. This report summarizes key points, gaps in knowledge, and recommendations resulting from this summit. A list of topics and summit attendees, who included speakers and representatives from a broad array of professional organizations and agencies, is available online (https://www.nanonline.org/NAN/_Research_Publications/Geriatrics_Summit_2.aspx).
IDENTIFYING OLDER ADULTS WITH COGNITIVE IMPAIRMENT
Despite increased prevalence of cognitive impairment, it remains underrecognized and undertreated.5 Primary care practitioners (PCPs), for example, do not detect as many as 60% of cases of dementia,5-7 which is defined as cognitive impairment severe enough to interfere with independent functioning. Detection rates are even lower for individuals who have early-onset dementia (that occurring before the age of 65 years), are Black or Hispanic, or have mild cognitive impairment (MCI), in which cognitive decline and impairment are measurable but not severe enough to interfere with ability to perform usual daily activities.5,8,9 Given the growing personal and societal burden of dementia, there is an urgent need to identify cases earlier so that interventions, including safety, care, and life planning, can be applied in milder disease states, when they might have the best chance of preventing avoidable crises or altering the trajectory of decline.10-12
Risk Assessment
The US Census Bureau predicts there will be almost 95 million people aged older than 65 years in 2060.13 There are only approximately 500,000 primary care physicians,14 however, indicating that screening the entire population every year within the context of primary care clinics is not feasible even if evidence reviewed by the US Preventive Services Task Force were sufficient to support the practice.15 For this reason, risk-based, or case-finding, approaches have been proposed to reduce the number of people to be screened.16 These approaches use risk assessment tools to identify individuals most likely to have cognitive impairment and then focus screening efforts on that subpopulation. Targeting individuals most likely to be affected improves the likelihood that a positive screening test result will reflect actual impairment and helps to optimize allocation of treatment resources.
Multiple models exist to predict risk of dementia,17 but only 2 tools have been developed with the explicit intent to predict risk for dementia in primary care clinics: the Brief Dementia Screening Indicator (BDSI)18 and the Rapid Assessment of Dementia Risk (RADaR).19 The BDSI is computed based on 7 patient characteristics, 5 of which (age, body mass index, history of stroke, presence of diabetes, and use of antidepressant medications) are generally available in patients’ medical records in primary care clinics; the 2 others (education and need for assistance managing money or medications) are variably represented. In 4 large well-characterized and diverse community- and population-based cohorts, a score of 22 or higher on the BDSI identified people aged 65 to 79 years having a dementia incidence over the subsequent 6 years similar to that of people aged 80 years or older; this group had a risk for dementia that was double that of same-aged people.18 The second risk measure, RADaR, was tested in 3 community-based cohorts. This tool, consisting of 5 questions—2 orientation items and recall of 3 words—demonstrated a combined accuracy (area under the curve) of 0.83 for predicting dementia over the subsequent 3 years.19 In that study, RADaR was slightly more accurate than the BDSI (area under the curve = 0.82 vs 0.72). Neither tool, however, was tested in actual primary care clinics—rather, they were evaluated in community- and population-based samples to simulate primary care populations.
Cognitive Screening
Characteristics of ideal cognitive screening tools for use in primary care clinics have been articulated previously and are summarized in Table 1.20,21 Although several patient- and informant-based measures have been recommended to detect dementia in primary care clinics and are listed in resource kits,21-25 these measures have not been shown to reliably detect milder stages of cognitive impairment (eg, MCI) or to distinguish them from dementia in primary care clinics. A recent meta-analysis found that the most frequently used cognitive screening test in primary care is the Mini-Mental State Examination (MMSE),26 which is outperformed by other tests and recommended to be replaced.27 A systematic review of short tools (requiring 2-5minutes; 12 tools), longer tools (requiring 5-20 minutes; 26 tools), and computerized tools (12 tools) in memory clinics and population-based cohorts revealed a lack of validation studies in MCI and AD samples for a majority of them.28 The Montreal Cognitive Assessment (MoCA)29 was the most well validated in memory clinics and 1 of only 2 measures validated in population-based cohorts, yet high rates of false-positives in important subpopulations (eg, individuals with low education or sensory impairment)30,31 limit its validity in heterogeneous groups of older adults.
Characteristics of Ideal Cognitive Screening Tools for Primary Care Clinics
In summary, most cases of MCI and frank dementia among older adults are not diagnosed by their PCPs, and these individuals do not receive interventions that may slow or prevent further decline or address other health risks related to cognitive impairment, such as poor compliance with medications or falls. Identification of patients at high risk for cognitive impairment is a viable first step that can be completed by extracting data from health records or by asking a few questions to narrow the pool for whom cognitive screening tests would be most likely to identify true impairment. Two risk assessment tools have been developed but have not been validated in primary care clinics; a third tool using electronic capture of health record data is currently being tested in a clinical trial.32 In contrast, several cognitive screening tests are available, but studies demonstrating that they have adequate levels of accuracy for detecting cognitive impairment, particularly MCI, in primary care clinics are lacking.
CHALLENGES TO IMPLEMENTING EARLY DETECTION STRATEGIES IN PRIMARY CARE CLINICS
In the United States, most physicians are employed by health systems or corporate entities.33 Changing clinical practice will therefore require optimizing workflow within these systems. The annual wellness visit (AWV) presents a promising avenue for implementing broader cognitive impairment screening. The law defining the AWV states that the visit should include “assessment of an individual’s cognitive function by direct observation, with due consideration of information obtained by way of patient report, concerns raised by family members, friends, caretakers, or others.”34 The law does not require or specify use of a screening tool, leaving those choices to the discretion of the clinician. The AWV is underused, however, with only 23% to 34% of those eligible having such visits.35,36
Even when AWVs take place, cognitive assessment occurs during fewer than 30% of these visits.37 The reasons for such low levels of cognitive assessment during AWVs include both time and knowledge constraints.38 To address knowledge constraints, training programs—such as Dementia Care Aware, the KAER program (Kickstart, Assess, Evaluate, Refer), and Project ECHO (Extension for Community Healthcare Outcomes)—are available and increase support for detection among clinicians by providing access to dementia experts and toolkits; some of these trainings offer simplified workflows for completing a basic dementia workup in primary care clinics. With regard to time constraints, short cognitive screening tests can be administered, scored, and interpreted by supervised clinic staff with minimal training and no prior experience and do not substantially disrupt workflow.39,40 Moreover, they are acceptable to patients and increase the likelihood of dementia diagnosis and referral to a dementia specialist.39,40 Studies have shown, however, that only 17% to 35% of people who screen positive receive dementia-related follow-up action by their PCP39-41 and, in one study, 66% declined memory specialist referral when it was offered for diagnostic assessment and follow-up.42
RECOMMENDATIONS FROM SUMMIT PARTICIPANTS
Clinical Approach
Screening the entire population of people aged older than 65 years annually is neither feasible nor currently supported by available evidence.15 Summit participants recommend that clinicians use a tool for risk stratification to identify a higher-prevalence pool to be screened. Because change relative to an individual’s past performance is less stigmatizing and more likely to be an early signal (vs identification of a behavior that has already become problematic), prescreening tools should focus on identifying changes in behavior and function rather than problems.
No one approach will meet all needs. Additional studies are required to tailor strategies for detecting cognitive impairment to the settings in which they are to be used. Particularly important are strategies that target health and care inequities among Black and Hispanic individuals, in whom cognitive impairment is less likely to be diagnosed when present and who are more likely to be misclassified as impaired by some cognitive screening tests.43 The capacity of primary care clinicians to respond effectively to cognitive impairment detected by screening—whether MCI or dementia—needs more research, as do strategies for linking screening outcomes to clinical care pathways.44 Research examining whether screening for behavioral changes could improve early detection is also needed. Importantly, future studies should use a validated MCI diagnosis as the gold standard instead of using another screening measure given that screening tools are not meant to be diagnostic and cannot replace a full clinical diagnostic evaluation. The ideal cognitive screening test would meet all the criteria listed in Table 1 and be automated, saving clinician time and avoiding human error in scoring and interpretation.
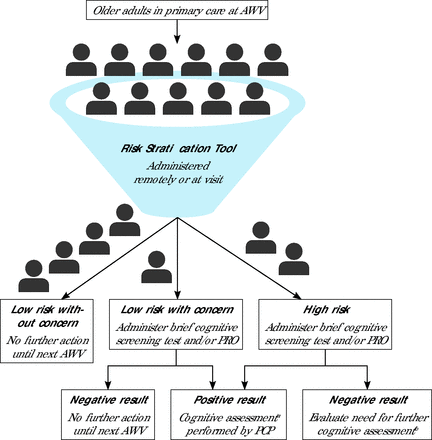
With these considerations in mind, summit participants recommend implementing the following workflow for early detection of cognitive impairment in primary care (summarized in Figure 1).
Recommended workflow for early detection of cognitive impairment in primary care.
AWV = annual wellness visit; PCP = primary care practitioner; PRO = patient- or informant-reported outcome (questionnaire).
aAssessment could be completed at the same visit or across several subsequent visits, and could include a validated cognitive screening measure (eg, Montreal Cognitive Assessment), assessment of possible contributing factors (eg, mental health, medications), and/or additional diagnostic testing (eg, neuroimaging, sleep study).
bCould include part or all the components listed in the above footnote.
(1) Identify individuals at high risk for cognitive impairment using a risk stratification tool having a small number of questions and/or using an alert in the electronic health record (EHR) based on information already available in the record.
(2) For patients having a concern or high risk, use a flag at this stage to trigger administration of a short cognitive screening measure (taking ≤5 minutes) by clinic staff and/or administration of a patient or informant questionnaire before the patient sees the PCP.25,45 Alternatively, a brief cognitive screening tool validated for remote administration could be deployed via the EHR.25
(3) If the screening result is positive, initiate a 3- or 4-visit assessment pathway.
The first visit could include administration of a longer cognitive screening measure, such as the MoCA, to confirm a positive screen and get a better sense of where difficulties might lie. Gathering information to rule out modifiable conditions that affect cognition (eg, sleep disorders, severe mental health problems) based on a brief first examination, assessing physical signs, and reviewing medications could also be part of the visit.
The second visit could focus on assessment of any changes since the first visit and provide time to make referrals for additional tests, such as a sleep study or neuroimaging.
The third visit could be spent reviewing results of tests and deciding whether referral to a dementia specialist is needed.
(4) Use practical, evidence-based guidance for making and communicating the diagnosis of a neurocognitive disorder and initiating treatment.46,47
Activating Stakeholders
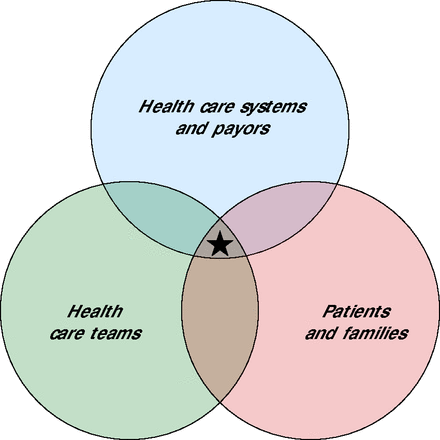
For successful implementation of such a risk assessment and screening strategy, 3 stakeholder groups need to be activated: health care systems, health care teams, and patients and their families (Figure 2).
Activating stakeholders to implement a workflow for early detection of cognitive impairment.
Note: To optimally implement a screening workflow, all 3 stakeholder groups must be incentivized. Ideally, incentives common to all 3 groups (denoted by the star) can be identified and serve to move the workflow forward.
One way to incentivize health care systems, including payers, is to alert them to the costs and consequences of dementia including discontinuity of care,48 preventable hospitalizations,49 readmissions,50 and mortality.51 Current levels of engagement between key payers and health care systems are not sufficient to problem-solve challenges and to identify incentives for screening and other procedures needed to diagnose cognitive impairment. Critical goals include eliminating carve-outs for behavioral health (payers’ exclusion of coverage for behavioral health services), fostering team-and value-based models of cognitive care,52,53 and doing away with competition for Medicare relative value units. Importantly, the behavioral health section of the Medicare physician fee schedule was changed in 2023 to allow provision of behavioral health services by nonphysician practitioners under general supervision instead of direct supervision only, and coverage of services via telehealth has been extended through 2024. These changes add flexibility and greatly extend access to cognitive assessment.
To activate health care teams, dementia specialists can partner with professional organizations focused on primary care to develop and/or refine existing materials to increase knowledge of and competence in procedures for identifying and comanaging cognitive impairment. In addition, practical guidance on billing and Current Procedural Terminology codes that can be used for these services and advocacy for a billing code specific to cognitive screening,25 like the one for depression screening (ie, G0444), will be helpful.
To activate patients and their families, it is important to communicate the message that there are reversible causes of cognitive impairment, and that dementia is a manageable chronic condition with many opportunities to benefit from early diagnosis and management,10,11 including, for some patients, recently approved anti-amyloid therapies specifically for early AD. Expanded public messaging around preserving brain health through managing modifiable risk factors and the importance of screening for early detection and care might be effective. Suggestions to “get the word out” include placing signs or leave-behind (“rack”) cards or pamphlets in public spaces, including physicians’ offices. Such material should always include actions patients can take, such as visiting a website or speaking to their clinician.
CONCLUSIONS AND FUTURE DIRECTIONS
With the expected increase in cognitive impairment due to population aging and with recent approvals of new drugs to treat early AD (and more in the pipeline54), creative solutions will be needed to help address an increased clinical workload, such as partnering with community health workers, libraries, and educational programs, and leveraging telehealth. Automated tools for risk stratification, cognitive screening, and follow-up on a positive result are already in various stages of validation,55,56 and models for successful implementation of screening procedures within EHRs of large health systems are available.57 Clinical trials within a variety of primary care clinics are needed to assess the feasibility and utility of multistep protocols that combine risk assessment approaches with short cognitive screening tests as case-finding methods that alert PCPs to consider further evaluation for cognitive impairment. Because not everyone has a PCP, similar studies will be needed in community settings, as well as urgent care clinics and emergency departments. Potential barriers to cognitive screening from the patient and family perspective are not well understood either and require further study.
Increased screening will lead to risk identification and need for follow-up. A cognitive care plan must be available to provide patients with support and access to necessary care throughout the spectrum of disease, especially in vulnerable populations of older adults such as minoritized groups who may have unique barriers to health care access. Care plans must address the key decision of whether the PCP will manage the patient alone, comanage with a specialist, or rely on the specialist for management. Although initial blueprints for collaborative practice between primary care and dementia specialists have been proposed58 and improvements in clinical outcomes of patients with dementia demonstrated,59 research delineating which patients should be referred, how much initial workup should be done before referral, and which groups should be managed long term by specialists will be beneficial. A step-by-step guide with practical information about documentation and billing codes and effective communication between care team members also may be helpful. To increase follow through, patients and their families need an explanation about the reason for referral and what the specialist can provide that the PCP cannot. Additional research on how to incentivize patients to follow up on positive screening results is an area needing further study.
In closing, we expect that the reporting of this Summit’s key points and recommendations will be a catalyst for developing partnerships between PCPs and dementia specialists and for creating concrete plans to facilitate early detection of cognitive impairment among older adults in primary care clinics and to implement procedures for following up on positive screens. Harnessing the collective wisdom of PCPs and dementia specialists in collaboration with patients, families, payers, health care administrators, and funders is imperative to move the needle toward reducing negative consequences of ADRD.
Acknowledgments
The authors would like to thank Tamara Golden, PhD for assistance with manuscript writing and are grateful for the insightful and constructive feedback on the manuscript received from Heather Snyder, PhD, Stefania Forner, PhD, and Malavika Tampi, MPH from the Alzheimer’s Association and Yalda Jabbarpour, MD and Michael Monroe, BS from the American Academy of Family Physicians. Finally, we extend our gratitude to each of the following organizations that supported the Summit by sending a representative (in alphabetical order): Alzheimer’s Association, American Academy of Family Physicians, American Association of Geriatric Psychiatry, American Association of Nurse Practitioners, American College of Emergency Physicians, American College of Physicians, American Geriatrics Society, American Psychological Association, Centers for Medicare & Medicaid Services, Collaborative Family Healthcare Association, Epic Systems, Inter Organizational Practice Committee, National Academy of Neuropsychology, National Institutes of Health/National Institute on Aging, US Department of Veterans Affairs, and US Preventive Services Task Force.
Footnotes
Conflicts of interest: authors report none.
Financial and material support: National Academy of Neuropsychology.
Disclaimer: The views expressed are solely those of the authors and do not necessarily represent official views of the authors’ affiliated institutions or the funder.
Previous presentation: Portions of this article were presented at the National Academy of Neuropsychology Geriatrics Summit 2; November 3-4, 2022; Denver, Colorado.
- Received for publication December 14, 2023.
- Revision received June 18, 2024.
- Accepted for publication August 5, 2024.
- © 2024 Annals of Family Medicine, Inc.