Abstract
PURPOSE We performed a pragmatic, cluster randomized controlled trial of a comprehensive practice-level, multistage practice transformation intervention aiming to increase behavioral health integration in primary care practices and improve patient outcomes. We examined associations between completion of intervention stages and patient outcomes across a heterogeneous national sample of primary care practices.
METHODS Forty-two primary care practices across the United States with colocated behavioral health and 2,945 patients with multiple chronic medical and behavioral health conditions completed surveys at baseline, midpoint, and 2-year follow-up. We examined effects of intervention on patient health and primary care integration outcomes using multilevel mixed-effects models, controlling for baseline outcome measurements.
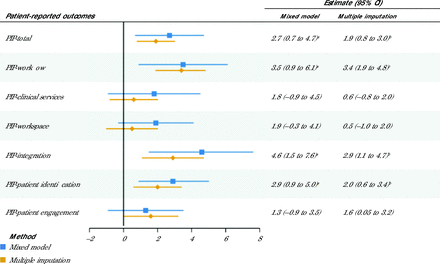
RESULTS No differences were found associated with the number of intervention stages completed and patient health outcomes including depression, anxiety, fatigue, sleep disturbance, pain, pain interference, social participation, and physical function. However, the completion of each intervention stage was associated with increases in Practice Integration Profile domain scores and confirmed with modeling using multiple imputation for the following: workflow 3.5 (95% CI, 0.9 to 6.1), integration methods 4.6 (95% CI, 1.5 to 7.6), patient identification 2.9 (95% CI, 0.9 to 5.0), and total integration 2.7 (95% CI, 0.7 to 4.7).
CONCLUSION A practice-centric flexible practice transformation intervention improved integration of behavioral health in primary care across heterogeneous primary care practices treating patients with multiple chronic conditions when accounting for completion of intervention stages. Interventions that allow practices to flexibly improve care have the potential to help complex patient populations. Future research is needed to determine how to best target patient health outcomes at the population level.
INTRODUCTION
Most patients with behavioral health problems receive behavioral care in primary care settings rather than specialty mental health settings,1,2 with a reported 15.9% of visits for patients seen in primary care settings addressing mental health needs directly.3 Primary care practices struggle to address these complex needs, with only 26% to 44% of primary care practices having a colocated behavioral health provider (BHP),4 although these numbers are increasing.5 Integrated behavioral health (IBH) is associated with improved access and engagement in mental health services, mental and physical health patient outcomes, and experience of care.6-10 Integrated behavioral health models vary,6,7 but they typically have a BHP, such as a psychologist or social worker, embedded in the primary care practice who works collaboratively with primary care clinicians to assess and manage behavioral health needs.11
Evidence-based IBH models of care are difficult to implement, given that they require complex practice-level changes customized to each practice, and interventions and effect vary.6,7,12-14 Best practices for exemplary integration have been identified that include a clear mission and focus on behavioral health, quality-improvement (QI) processes, defining clear staff and clinician roles, and a team-based approach.15 Practice facilitation, lean management approaches, and learning collaboratives have specifically been shown to facilitate IBH implementation.16-18
We tested a pragmatic, cluster randomized, controlled trial to evaluate a comprehensive practice-level intervention to improve behavioral health integration and patient outcomes in primary care practices, specifically targeting patients with multiple (≥2) chronic medical and behavioral health conditions. We randomized primary care practices across the United States to either a control arm of IBH services as usual vs a 24-month intervention arm that tested a multistage practice-based intervention informed by a lean management toolkit, a structured redesign method to improve IBH with optional process improvement workbooks, QI coaching, clinician and staff education, and collaborative learning. We hypothesized that practices that completed more stages in the intervention arm would report greater levels of integration, and patients in these practices would report greater improvement in their physical and mental health over time.
METHODS
Study Design
To test the intervention, we randomized primary care practices to 1 of 2 arms within a large-scale, pragmatic, cluster randomized, clinical trial. We compared the active intervention arm, which included a toolkit-based implementation strategy to increase the degree of IBH, with the IBH services as usual arm. A detailed description of the study protocol is published elsewhere,19 and the study is registered at ClinicalTrials.gov (NCT02868983) and was approved by the University of Vermont Committee on Human Research in the Medical Sciences (CHRMS #16-554) and institutional review boards at other participating locations.
Practices and Participants
Eligible primary care practices were required to have an existing employed and colocated BHP of ≥0.5 full-time equivalent (FTE), actively bill Medicare and other insurers for BHP services, use a shared electronic health record system, and score <75 of 100 on the Practice Integration Profile (PIP)20 to ensure that room for improvement in integration was possible. Eligible patients either had ≥1 chronic medical condition and ≥1 chronic behavioral health condition or ≥3 chronic medical conditions.
Integrated Behavioral Health and Primary Care Intervention
Practices randomized to the active intervention group (IBH and primary care [IBH-PC]) were provided with the IBH-PC toolkit, which included the following components: (1) workbooks to guide the QI project, (2) online education tailored to practice personnel roles (clinician, BHP, nurse, etc), (3) an online learning community, and (4) remote coaching for the primary care practice’s QI team facilitator and team by a trained QI professional paired with a psychologist familiar with IBH. Portions of the toolkit were iteratively developed in prior studies.16,21-23 In keeping with the pragmatic design of the present study, each team tailored its use of the toolkit according to the needs of the practice including determining when to start within a 2-year timeframe and which components of the intervention to use.
The toolkit was presented in workbooks according to the following stages to organize the QI team’s activities into discrete steps to move toward more integration of primary care and behavioral health services: Stage 1–Planning; Stage 2–Redesign of workflows, and Stage 3–Implementation of practice changes. The education, online learning, and remote coaching components were offered throughout the 3 stages and were accessed as needed by each QI team. Each stage included a set of steps for QI teams to follow, and coaches confirmed progress across steps and assisted in adaptations to the intervention to best meet the team’s goals as teams requested coaching support. Coaches documented completion of each step or completion of an adapted step. For example, an early step in Stage 1 (Planning) was “Develop your vision of IBH,” when an intervention-arm practice chose to review and revise a recently developed vision statement regarding IBH, after which the coach documented that step as completed. Coaches met weekly as a group to review practice progress and come to consensus on the coding of step completion, which was documented in a shared record. Practices that had completed all steps in a stage were considered to have completed that stage for purposes of analysis. Practices that chose to skip ≥1 steps were reassessed by coaches separately on the basis of coaching notes and then cross-compared and finalized in follow-up coaching team meetings to reach final consensus on stage completion status. If ≥1 steps in a stage was assessed as skipped, that stage was deemed to be incomplete.
Measures
All measures were administered by surveys to practice and patient participants at baseline, midpoint, and the 2-year follow-up.19 Participants provided baseline data directly after practice randomization or patient recruitment, midpoint data at approximately 12-18 months after baseline, and 2-year data at approximately 21-27 months after baseline.
Patient Heath Outcomes
The 29-item Patient-Reported Outcomes Measurement Information System (PROMIS-29 [version 2.0]) measured patients’ physical function, anxiety, depression, fatigue, sleep disturbance, social participation, and pain interference in the past 7 days using a 5-point response option for each separate scale.24,25 An additional pain numeric rating scale (0-10) was included in which a higher number indicated more intense pain. The PROMIS-29 items were also used to create composite scores of mental and physical health summary scores.26 Responses were scored on a T-score metric based on the PROMIS normative reference sample of US adults; scales were scored with a mean of 50 and an SD of 10. A higher T-score indicated worse severity of anxiety, depression, fatigue, pain interference, pain intensity, and sleep disturbance. A lower T-score indicated worse severity of physical function and social participation. Scores ≥3 from 50 indicated at least mild impairment. Depression was measured with the 9-item Patient Health Questionnaire (PHQ-9), a self-administered screening tool for assessment of the severity of depressive symptoms; the PHQ-9 has good reliability (α = 0.89).27 Anxiety was measured with the 7-item Generalized Anxiety Disorder (GAD-7) self-report scale, which identifies probable cases of GAD; the GAD-7 also has good reliability (α =0.83).28
Practice Integration Outcomes
The PIP (version 1.0) was developed by a national team of clinicians and clinical researchers and operationalizes the lexicon for behavioral health and primary care integration.29 The PIP has been shown to discriminate differing levels of integrated care processes and differences in type of practice.20 The 30-item PIP was administered to ≥4 people at the practice (ie, medical primary care clinician, BHP, an administrator such as clinic manager, and clinician or staff of the practice’s choice). The PIP assessed levels of the practice’s behavioral health integration across the following 6 domains: practice workflow, clinical services, integration methods, case identification, patient engagement, and workspace arrangement and infrastructure. For each domain, the score ranges from 0 (no integration) to 100 (full integration). The total integration PIP score is the unweighted average of the 6 domain scores.20,30,31
Statistical Analysis
We ran multilevel mixed-effects models using the number of intervention stages completed as the primary exposure of interest. Baseline outcome measurement, as well as the time interval from baseline to midpoint and follow-up measurements, respectively, was adjusted for in all analyses.
Patient Health Outcomes Analysis
We evaluated the association between the number of IBH-PC intervention stages completed and patient-reported outcomes using 3-level mixed models with repeated (midpoint and 2-year follow-up) measurements (level 1) nested in patients (level 2) nested in individual primary care practices (level 3). We modeled patient and practice as random effects. Each model included 2 random intercepts to account for the difference in average outcome at individual and practice levels. For all outcomes, only patients with room for improvement were analyzed (ie, baseline measures for PROMIS-29 were ≥2 points worse than the T-score of 50; PHQ-9 ≥10; GAD-7 ≥10). We adjusted for age, sex, race, ethnicity, employment status, living region (urban/rural), and insecurity status (ie, noted as present if ≥1 food, housing, or financial deprivation was reported). Socioeconomic disadvantage was included as a binary variable (ie, present, not present) if food, housing, or finance was insecure.
Practice Integration Outcomes Analysis
We assessed the association between the number of IBH-PC intervention stages completed and PIP total and scale scores using 3-level mixed models with repeated (midpoint and 2-year follow-up) measurements (level 1) nested in staff/providers (level 2) nested in primary care practices (level 3). Staff/provider and practice were modeled as random effects. Each model included 2 random intercepts to account for the difference in average PIP score at individual and practice levels. We adjusted for the ratio of BHP FTE:primary care clinician FTE and baseline outcome measurement, as well as the time interval from baseline to midpoint and follow-up measurements, respectively.
Multiple Comparison Corrections
We considered correction for 8 multiple comparisons for PROMIS-29 outcomes and 6 multiple comparisons for PIP outcomes. The following 2 procedures were considered: Bonferroni correction to control for a familywise type I error rate of 0.05, and the Benjamini-Hochberg method to control the false-discovery proportion at the 0.05 level.
Sensitivity Analysis
To test the robustness of the results, we performed multiple imputations by chained equations using the mice function in R (R Project for Statistical Computing),32 in addition to the primary analysis using mixed models, to handle missing data across 3 time points caused by participant nonresponse. We used 25 imputations and 30 iterations to predict missing data values. The intervention effects on patient-reported outcomes and practice integration were assessed using the methods described above, with exclusion of 1 practice that did not complete any intervention stages.
RESULTS
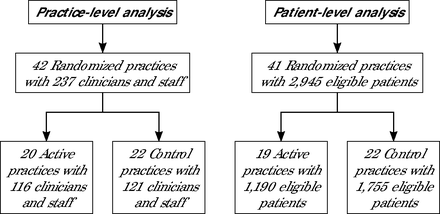
A total of 42 practices were randomized, with 1 practice unable to provide eligible patient data and therefore not included in the patient-level analysis (Figure 1). All primary care clinicians and staff (n = 237) and patients (n = 2,945) completed baseline and ≥1 of the follow-up assessments. Patients had an average age of 61.8 years, were mostly female (64.0%), and had an average of 4.4 chronic conditions at baseline (Table 1). Some patient baseline characteristics related to race, employment, annual household income, diabetes, urban/rural area, and food insecurity differed significantly between study arms. The primary care practices were predominantly nonprofit organizations (88%) located in urban areas (83%) and had no significant differences in practice characteristics between arms (Table 2). Among the 20 primary care practices randomized to the intervention arm, 13 (65%) completed all 3 intervention stages, 6 (30%) completed 2 stages, and 1 (5%) did not complete any stage. The 22 primary care practices randomized to the control arm completed 0 intervention stages, as expected.
CONSORT Diagram
CONSORT = Consolidated Standards of Reporting Trials.
Patient Characteristics and Outcomes at Baseline
Practice Characteristics and Outcomes at Baseline
Patient Health Outcomes
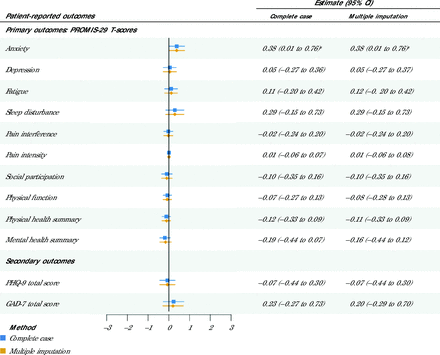
Patients with multiple chronic conditions in the intervention arm did not report significantly different outcomes compared with patients in the IBH services as usual arm. No significant association was found between the number of intervention stages completed and patient health outcomes (Figure 2), consistent with the sensitivity analysis, with the exception of the PROMIS-29 anxiety subscale, which was slightly greater for the intervention arm but not clinically meaningful. These analyses did not account for amount of service accessed by medical or behavioral health clinicians at each practice.
Adjusted Effect of Intervention Stage Completion on Patient Health Outcomes
GAD-7 = Generalized Anxiety Disorder 7-item scale; PHQ-9 = Patient Health Questionnaire 9-item scale; PROMIS-29 = Patient-Reported Outcomes Measurement Information System-29.
Note: P values are not corrected for multiple comparisons. Positive estimates indicate that the completion of each additional intervention stage is associated with a higher outcome score; negative estimates indicate that the completion of each additional intervention stage is associated with a lower outcome score.
a P < .05.
Practice Integration Outcomes
Primary care practice personnel in the intervention arm reported significantly greater integration based on the PIP compared with the practices in the IBH services as usual arm (Figure 3). With mixed-model analysis, the completion of each additional intervention stage was associated with a significant increase in PIP score of 3.5 (95% CI, 0.9 to 6.1) for workflow, 4.6 (95% CI, 1.5 to 7.6) for integration methods, 2.9 (95% CI, 0.9 to 5.0) for patient identification, and 2.7 (95% CI, 0.7 to 4.7) for total integration PIP score. After implementing multiple imputation, result patterns remained consistent. These findings remained the same after multiple comparison corrections.
Adjusted Effect of Intervention Stage Completion on Integration Level
PIP = Practice Integration Profile.
Note: P values are not corrected for multiple comparisons. Positive estimates indicate that the completion of each additional intervention stage is associated with a higher outcome score; negative estimates indicate that the completion of each additional intervention stage is associated with a lower outcome score.
a P <.05.
b P <.01.
c P <.001.
DISCUSSION
A QI toolkit designed to allow practices to define practice-centric targets for improvement offered an effective method for busy, complex primary care practices to significantly increase their overall level of integration, as well as across workflow, integration methods, and patient identification domains. Practice-level changes were complex and practice driven, and sustainability was likely interrupted by the COVID-19 pandemic at the end of the observation period (May to December 2020). Practice-centric approaches to implementing IBH helped practices improve their level of integration. Substantial investments in time and resources are likely necessary for implementation of substantial and sustainable change.
Improvements in self-reported patient health outcomes across a random sample of patients with multiple chronic medical and behavioral health conditions were not observed, even when limiting analyses to selected patients whose baseline scores indicated room for improvement. Improvements might not have been identified because analyses did not select or measure engagement in direct care by BHPs (ie, patients might not have received any direct treatment from the BHP), and patients might have already received treatment for their chronic conditions at the time of baseline measurement. Future studies are needed to evaluate whether patient outcomes improve for patients who have received care directly from colocated BHPs. Future research is needed to discover how and what changes practices make with this intervention. Changes might include foundational or structural changes that are critical path precursors before patient outcomes show improvement. Future research is also needed to investigate the association between specific clinical operational changes with improvements in patient service utilization and targeted patient health outcomes.
The success of the intervention was likely due to the practice-centric approach that allowed practices to flexibly set goals they deemed appropriate to their settings, using evidence-based QI methods and targeting a defined group of patients with multiple chronic medical and behavioral health conditions. Practices were able to designate their own intervention teams, meet on their own choice of schedule and frequency, and engage materials and resources as they saw fit to make real changes in their practices. Given the variation in primary care practices’ readiness and capacity for change, as well as the heterogeneity inherent across these practices’ processes and structures, practice-centric interventions that balance flexibility with consistent structure such as this intervention might help decrease barriers to disseminating IBH. The COVID-19 pandemic had a profound effect on the final year of the observation period. The practice findings of improved integration might have been more robust without the pandemic disruption, given practices’ need to pivot to address the crisis. The demand to address behavioral health needs has increased since the pandemic. Given that evidence-based behavioral interventions are useful for the management of chronic diseases, and the burden of chronic disease is growing, there is reason to believe the relevance of IBH will continue to grow.33
Changes in integration did not affect clinical services, workspace, or patient engagement domains on the PIP. The lack of improvement in these areas of integration might be because these areas are relatively more demanding or require more time for practices to implement change, given that they require changes in staffing and/or skills, as well as the comfort of patients with multiple chronic conditions in self-management. In addition, practices might have been less inclined to make specific goals related to these areas given their challenging nature. Further, it should be noted that workspace was rated at baseline as the highest domain of integration (Table 2; 87.6 of 100) and had minimal room for improvement.
Primary care practices have struggled to adopt IBH for various reasons such as workforce supply issues, including a shortage of BHPs available and willing to work in primary care settings, unstable reimbursement and funding models, little adaptation of behavioral health into practice workflow, and the always present difficulty creating and sustaining practice change.5,34,35 Policy changes, such as the Patient Protection and Affordable Care Act, have caused increases in volume of care within community health clinics and other primary care settings and an increased focus on complexity of the behavioral health issues in need of care in these settings. The COVID-19 pandemic complicated delivery of primary care via social distancing and exacerbated issues of loneliness, anxiety, and insomnia along with mental health acuity.36-38 Results of our intervention show that despite practices being flexible in how and when they approach and target practice change, they can nimbly advance IBH when given the right support at a time when patient needs are increasing.
Limitations of the present study include recruitment of practices that already had an established history of integrating behavioral and primary care and scored in the middle range of self-reported IBH as assessed by the PIP. Whereas we did not test whether our intervention is effective with lower levels of integration or with practices just beginning to add BHPs to their practice teams, the flexible nature of this intervention might benefit those practices as well. We also did not account for the quality and quantity of medical care access each patient received, whether the patient was treated specifically by the BHP in the practice, or whether the patient was in need of behavioral interventions at baseline. Future studies are needed with careful patient selection to examine the effect of improving IBH on these complex patient populations.
Primary care practices face an unprecedented challenge in the high demand for care to address the complex needs of patients with multiple chronic conditions. A practice-centric flexible intervention aimed at improving the level of IBH in primary care can help practices transform to meet these needs and improve the health of their most complex patients. Future research is needed to determine how to leverage patient data collected in routine care (ie, via electronic health record systems) to enable careful evaluation of the effect of these primary care transformations and ensure that pathways to dissemination of IBH are supported.
Acknowledgments
The authors acknowledge the work of the Integrating Behavioral Health and Primary Care (IBH-PC) Research Team in the implementation of this intervention study. This research was supported by the Patient-Centered Outcomes Research Institute (PCS-1409 to 24372), the University of Washington (UW) Institute of Translational Health Sciences (UL1 TR002319), and the UW Primary Care Psychiatry/Behavioral Health Integration Fellowship T32 (T32MH020021).
Footnotes
Conflicts of interest: authors report none.
- Received for publication October 31, 2023.
- Revision received August 10, 2024.
- Accepted for publication October 10, 2024.
- © 2025 Annals of Family Medicine, Inc.