Abstract
PURPOSE Postpartum depression affects up to 22% of women who have recently given birth. Most mothers are not screened for this condition, and an ideal screening tool has not been identified. This study investigated (1) the validity of a 2-question screen and the 9-item Patient Health Questionnaire (PHQ-9) for identifying postpartum depression and (2) the feasibility of screening for postpartum depression during well-child visits.
METHODS Study participants were English-literate mothers registering their 0- to 1-month-old infants for well-child visits at 7 family medicine or pediatric clinics. They were asked to complete questionnaires during well-child visits at 0 to 1, 2, 4, 6, and 9 months postpartum. Each questionnaire included 2 depression screens: the 2-question screen and the PHQ-9. The mothers also completed the depression component of the Structured Clinical Interview for DSM-IV (SCID) initially, and again at a subsequent interval if either screening result was positive for depression.
RESULTS The response rate was 33%. Of the 506 women who participated, 45 (8.9%) had major depression (ie, they had a positive result on the SCID). The screen sensitivities/specificities over the course of the study were 100%/44% with the 2-question screen, 82%/84% with the PHQ-9 using simple scoring, and 67%/92% with the PHQ-9 using complex scoring. In addition, the corresponding values for the first 2 items of the PHQ-9 (ie, the 2-item Patient Health Questionnaire or PHQ-2) were 84%/79%. Some 38% of women completed their 2- to 6-month questionnaires during well-child visits; the rest completed them by mail (29%) or telephone (33%).
CONCLUSIONS The 2-question screen was highly sensitive and the PHQ-9 was highly specific for identifying postpartum depression. These results suggest the value of a 2-stage procedure for screening for postpartum depression, whereby a 2-question screen that is positive for depression is followed by a PHQ-9. These screens can be easily administered in primary care clinics; feasibility of screening during well-child visits was moderate but may be better in clinics using a mass-screening approach.
- Depression
- postnatal depression
- postnatal care
- postpartum period
- screening
- postpartum depression
- preventive health services
- PHQ-9
- primary care
- practice-based research
INTRODUCTION
Postpartum depression is increasingly recognized as a unique and serious complication of childbirth, with an estimated prevalence in the 12-month postpartum period of up to 21.9%.1 Mothers’ depressive symptoms—diminished mood, pleasure, energy, concentration, and self-worth; psychomotor retardation; changes in appetite and sleep; and suicidal ideation—can markedly impair their sense of well-being, marital and other key relationships,2 work performance and productivity,3 relationships with their infants,4 and infants’ behavioral and cognitive development.5 Recognizing the seriousness of this disorder, the US Preventive Services Task Force has recommended routine depression screening for adults in practices that have systems in place to ensure accurate diagnosis, effective treatment, and follow-up.6 Most primary care practices do not have such systems in place, for either general or postpartum depression, however.
Studies of postpartum depression screening demonstrate that it is feasible in outpatient clinical settings, either during mothers’ postpartum visits7,8 or during infants’ well-child visits,9–11 with the use of screens such as the Edinburgh Postnatal Depression Scale7–10 or the 2-item Patient Health Questionnaire (PHQ-2).11 In 2 recent studies in which maternal depression screening was performed during well-child visits conducted in pediatric offices, mothers’ response rates were 55% to 74%.10,11 Two additional large studies involving a total of 860,479 mothers found that more than 80% said they were comfortable with the idea of being screened for postpartum depression.12,13 Unknown is the degree of comfort with screening among the 41% to 48% of women who did not respond to the questionnaire, or the level of compliance with screening among those who say they are comfortable. In reality, fewer than 50% of women with infants are currently being screened for postpartum depression.14–16 Several studies have confirmed that informal assessment or nonassessment for postpartum depression identifies fewer than one-half of cases or potential cases.7,8,17,18
Although physicians generally agree that it is important to recognize and treat postpartum depression, actual screening activities are quite variable. In a survey of members of the Washington Academy of Family Physicians, 70% (254 family physicians) said they always or often screened for postpartum depression at the postpartum examination, while 46% did so at well-child checkups; however, only 31% of those reported using a validated tool for screening.15 Two national surveys of pediatricians found that most do not feel well trained in postpartum depression or confident in their abilities to diagnose the disorder.19,20
Clinicians who screen for postpartum depression need a valid, reliable instrument. Two recent reviews evaluated several postpartum depression screens, including the Beck Depression Inventory, Bromley Postnatal Depression Scale, Center for Epidemiological Studies Depression Scale, Edinburgh Postnatal Depression Scale, General Health Questionnaire, Inventory of Depressive Symptomatology, Postpartum Depression Screening Scale, and Zung Self-Rating Depression Scale.1,21 These reviews found that most of the studies on postpartum depression screens were too small to effectively validate the screens, and external validity was often poor to fair. A third review looked at prenatal screening for women at risk for postpartum depression, using the Edinburgh Postnatal Depression Scale, standardized diagnostic psychiatric interviews, or both; this review concluded that none of the instruments evaluated met the criteria for routine prenatal application.22 Additional studies with large, representative samples are therefore needed to identify the ideal postpartum depression screen.
Other promising screens not covered in these reviews are the 9-item Patient Health Questionnaire (PHQ-9) and the 2-question screen. The PHQ-9, thought by some experts to be “the best available depression screening tool for primary care,” 23 consists of the Diagnostic and Statistical Manual, Fourth Edition (DSM-IV) criteria for major depressive disorder and uses a Likert scale response.24 When evaluated in a sample of 6,000 patients seen in 8 primary care clinics and 7 obstetrics-gynecology clinics, the PHQ-9 had a sensitivity and specificity of 88% (at a cutoff of ≥10), based on a structured interview.24 In a recent study of postpartum depression, the PHQ-9 correctly identified 4 of 13 cases, compared with 8 of 13 and 12 of 13 identified by the Edinburgh Postnatal Depression Scale and the Postpartum Depression Screening Scale, respectively. This study was limited, however, by its small number of depressed women (n = 13) and use of the formal depression interview only in women whose findings were positive for depression.25
The 2-question screen asks about the 2 fundamental symptoms of depression, diminished mood and pleasure, and has simple yes/no responses. In 2 separate large studies, this screen was found to have a sensitivity of 96% and a specificity of 57% to 78%.26,27 The addition of a help question, which asks whether “this is something with which you would like help,” increased the specificity to 94%.27 The PHQ-2, which consists of the first 2 questions of the PHQ-9, is similar to the 2-question screen except for its Likert scale response.28 Neither the 2-question screen nor the PHQ-2 has been validated in a purely postpartum population.
The purpose of this study was to determine the validity of the 2-question screen and the PHQ-9 in a sample of postpartum women, and to investigate the feasibility of screening for postpartum depression during well-child visits in family medicine and pediatric clinics. This validation study was conducted within the context of a randomized controlled trial, the overall purpose of which was to begin to assess the impact of stepped care treatment on postpartum depression.
METHODS
Study Overview
Before its inception, the study was approved by the University of Minnesota’s institutional review board. Study participants were recruited over a 12-month period, from October 1, 2005, through September 30, 2006. Mothers registering their infants for an initial well-child visit at 0 to 1 month of age at any of 7 participating clinics were given an enrollment packet consisting of a brief description of the study, an enrollment form (on which they were asked to indicate their willingness to participate), a consent form, and an initial questionnaire. Participants were given follow-up questionnaires at subsequent 2-, 4-, and 6-month well-child visits (or, if they were unable to complete them at these visits, were offered telephone or mailed questionnaires), and were mailed a final questionnaire at 9 months postpartum. Participants were also asked to complete the Structured Clinical Interview for DSM-IV (SCID) interview within 2 weeks of completing the initial questionnaire, and subsequently if they had not been previously depressed, and developed a positive screening result on a follow-up questionnaire.
Study Participants
Participants were recruited from 7 Minneapolis and St. Paul metropolitan area clinics. The clinics represented a diverse cross-section of patients, as the 4 family medicine residency clinics served primarily urban, ethnically diverse, low-income groups, whereas the 3 pediatric private clinics saw mostly suburban, white, mid- to upper-income patients. To enroll in the study, mothers needed to be English literate, be aged 12 years or older, and have a 0- to 1-month-old infant who received care at any of the participating clinics.
Measures
The initial questionnaire collected demographic information about the women: age, education, race/ethnicity, total family income, health insurance, marital status, number of children, and delivery date. Both the initial and the follow-up questionnaires contained the 2 depression screens (Table 1⇓): the 2-question screen, which consists of the 2 fundamental symptoms of depression (diminished mood and pleasure), and the PHQ-9, which contains the DSM-IV criteria for major depressive disorder.24 The first 2 questions of the PHQ-9 constitute the PHQ-2,28 which was also evaluated.
Depression Screens: 2-Question Screen and PHQ-9
All participants were also asked to complete the depression component of the SCID29 at 0 to 1 month postpartum and again later if they were previously not depressed but had a positive screen result on a follow-up questionnaire. The SCID interview, our reference standard for diagnosing major depression, evaluates the presence and frequency of 9 symptoms of depression, as well as other potential causes of these symptoms (eg, grief), and the result is scored as positive or negative. The SCID interviews were conducted by doctoral-level psychology students, whose training consisted of observing SCID training tapes and completing 5 practice tapes under the supervision and review of a highly experienced doctoral-level assessor, followed by weekly quality assurance assessment conferences throughout the study. Sixty-eight (13.4%) of the women could not be reached for the initial SCID interview; an additional 9 women could not be reached for a follow-up SCID interview.
Statistical Analysis
We scored the 2-question screen results as positive if the respondent answered “yes” to either or both of the 2 questions. Validity of the PHQ-9 was assessed using 2 separate scoring methods. For the simple scoring method, we summed the responses and considered a total score of 10 or greater to be positive, based on the cutoff of 10 or greater used in the previously cited PHQ-9 validation study.24 The complex PHQ-9 scoring method, modeled after the DSM-IV definition of depression,30 requires the presence of 5 or more symptoms, including symptom 1, symptom 2, or both, and each symptom must have a response of 2 or 3 with the exception of symptom 9, for which a response of 1 to 3 was acceptable. Given that the first 2 questions of the PHQ-9 constitute the PHQ-2, we also tested the validity of the PHQ-2; the result was considered to be positive if either question had a response of 2 or greater.
We conducted 2 sets of validity tests (including sensitivity, specificity, negative predictive value, and positive predictive value) for each screen. The first set was performed using only data from the questionnaire completed at 0 to 1 month, whereas the second set was performed using data from the entire study period. For the second set, a woman was said to have a positive 2-question screening result if her result was positive at any time point during the course of the study, and a negative screening result if her result was negative at every time point throughout the study.
To determine whether women who dropped out of the study (did not complete the final questionnaire) differed from women who completed the study, we used bivariate analyses (t tests and χ2 tests) to compare these 2 groups on maternal age, number of children, marital status, education, family income, and positive PHQ-9.
RESULTS
Participants
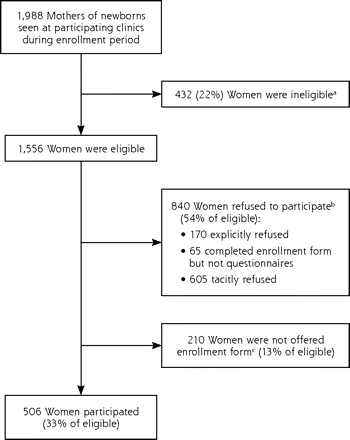
A total of 506 women participated in the study, which represented approximately 33% of the estimated 1,556 eligible women (response rate, 28% for family medicine clinics and 36% for pediatric clinics). Numbers of eligible women were based on the estimated number of English-literate mothers of newborns seen at participating clinics during the enrollment period. The reasons for nonparticipation among eligible women are shown in Figure 1⇓.
Numbers of eligible and participating women.
a The number of ineligible women was based on estimates of non–English-literate women at each site.
b The number of women who refused participation was based on an estimated refusal rate of 54%, seen in a prior randomized controlled trial conducted in the same general location.31 Included in this group are 170 who explicitly refused (as stated on their enrollment form), 65 who completed an enrollment form but not the questionnaire, and an estimated 605 women who tacitly refused to participate (they did not explicitly state that they refused but demonstrated their unwillingness to participate by not returning a completed enrollment form).
c The estimated number of eligible mothers not offered an enrollment form was calculated by subtracting 506 mothers who participated and 840 mothers who refused from 1,556.
Participants’ demographic characteristics, displayed in Table 2⇓, indicate that the sample was diverse with varied ethnicity and a broad range of income and education. Overall, 167 (33%) of participants were recruited from family medicine clinics, and 339 (67%) were recruited from pediatric clinics.
Participants’ Demographic Characteristics (N=506)
Only 34 (6.7%) of the 506 participants dropped out of the study (did not complete the final questionnaire). When women who dropped out were compared with those who completed the study, the dropouts were found to be younger and less educated, had more children, were less likely to be married, had lower family incomes, and were more likely to be depressed (Table 3⇓).
Comparisons Between Women Who Completed the Study and Women Who Dropped Out
Postpartum Depression Diagnosis
Twenty women (4.6%) had a diagnosis of major depression (ie, had a positive SCID result) at 0 to 1 month postpartum, and a total of 45 (8.9%) had a diagnosis of major depression over the entire course of the study.
Validity of the Depression Screens
Validity test results for the 2-question screen, the PHQ-2, and the PHQ-9 at the initial visit (0–1 month postpartum), using the SCID interview as the reference standard, are displayed in Table 4⇓. The highest sensitivity (100%) was seen with the 2-question screen, and the highest specificity (94%) was seen with the PHQ-9 using complex scoring.
Validity of the Depression Screens at 0 to 1 Month Postpartum (n = 438 Women, 20 With Major Depression)
Validity test results over the entire study period (0 to 9 months postpartum) are displayed in Table 5⇓ and confirmed high sensitivity of the 2-question screen and high specificity of the PHQ-9 with complex scoring. Negative predictive values were very high (97%–100%), whereas positive predictive values were modest (15%–43%), as one might expect from this population wherein only 9% of women had a formal diagnosis of major depression.
Validity of the Depression Screens Over Entire Course of Study (N = 506 Women, 45 With Major Depression)
We also considered the validity of a 2-step screening procedure as a whole over the 9-month follow-up period. In this analysis, we defined a positive result as concurrent positivity on both the 2-question screen and the PHQ-9; all other combinations were considered to be negative. With this approach, the procedure had a sensitivity of 80% (36 of 45), a specificity of 85% (392 of 461), a negative predictive value of 98%, and a positive predictive value of 34%.
Feasibility of Screening at Well-Child Visits
During the course of the study, 38% of participants completed their 2- to 6-month follow-up by completing the depression screens during well-child visits, 29% did so by mailed questionnaire, and 33% did so by telephone. Women enrolled from pediatric clinics were more likely to complete their questionnaires at the clinic site than were women enrolled from family medicine clinics (46.3% vs 27.2%, P = .000). Rates of in-office completion of the follow-up questionnaire were higher (46%) before August 1, 2006. After this date, incorporation of the electronic medical record in the family medicine clinics made it impossible to physically prepare participants’ charts with questionnaires before their visit, so in-office completion from these clinics thereafter declined.
DISCUSSION
Using the SCID interview as the reference standard, we found the 2-question screen to be highly sensitive (100%) for identifying postpartum depression, meaning that it did not miss any cases of postpartum depression—a desirable characteristic of an initial screening tool. In contrast, we found the PHQ-9 to be highly specific (92%–94%) for identifying postpartum depression, indicating a low false-positive rate in the overall sample—a desirable characteristic of a diagnostic tool. These results suggest the utility of a 2-stage screening procedure for postpartum depression, whereby the 2-question screen is used as the initial screening test and the PHQ-9 is used as a confirmatory test. Busy primary care professionals would undoubtedly find these 2 screens to be easier to administer than the formal, lengthier SCID interview, which requires additional specialized training.
An important benefit of this 2-stage approach is that many false-positive 2-question screen results (n = 258/506), likely more problematic in the early postpartum period when “the blues” are common, could be sorted out with the PHQ-9. False-positives (69/461 = 15% with the 2-step procedure) could be further identified during the initial visit for depression, and women with mild symptoms might initially be observed rather than treated. Clinicians also need to beware of false-negatives (9/45 = 20% with the 2-step procedure) and might advise women whose findings are positive by the 2-question screen and negative by the PHQ-9 that they need to contact their clinician if depressive symptoms persist and interfere with their function. Perhaps of even greater concern, though, is the risk of missing postpartum depression in women who do not bring their child in for well-child visits, as prior research has shown a relationship between maternal depressive symptoms and noncompliance with well-child visits.32
Although only 38% of the 2- to 6-month follow-up questionnaires were completed during well-child visits, the percentage was higher (46%) for questionnaires completed before August 1, 2006, when family medicine patient records were still on paper charts instead of electronic ones. Conversion to an electronic record made it impossible to physically prepare charts with screening forms before the visit. Our previous recruitment procedure was thus incompatible with the new electronic medical record, resulting in a decreased rate of completion of questionnaires in the office. Although we found the change to electronic records to be a deterrent to paper-and-pencil screening in our study wherein not all mothers were participants, routine screening via the electronic record would likely prove to be advantageous for a mass screening program that included all mothers.
The rate of completion of questionnaires in the clinic was greater in pediatric than in family medicine clinics (46% vs 27%). This difference is likely explained by the fact that the receptionists in the family medicine clinics appeared to have a larger number and range of responsibilities (eg, patients of all ages, with a greater variety of forms and needs), and usually appeared to be busier and proportionately less well staffed. These observed specialty differences would also help to explain why our in-clinic questionnaire completion rate was lower than the 55% to 74% rate of depression screen completion in 2 pediatric office-based studies.10,11 Future studies with family medicine clinics may need to explore incentives for both clinic personnel and potential participants to increase response rates. Also, it is possible that a mass screening program would be more readily incorporated into busy family medicine clinics, as busy receptionists would not be required to differentiate participants from nonparticipants.
Although relatively few women dropped out of the study, the comparison between dropouts and completers revealed a significantly higher rate of depressive symptoms (positive PHQ-9 results) among the dropouts. If this finding is validated in future studies, subsequent investigators may want to consider participant retention strategies tailored to the demographics of the dropouts (eg, being single).
Strengths of this study include the validation of 2 depression screens—the 2-question screen and the PHQ-9—in a postpartum sample, the longitudinal study design, use of the SCID interview as a reference standard for depression diagnosis, inclusion of both family medicine and pediatric clinics, and sample diversity. The study also has some weaknesses. For example, the modest initial participation rate (33%) is likely due to a combination of factors, such as mothers’ lack of time or attention, their reluctance to participate in a treatment trial, and busy receptionists’ not offering women enrollment forms and questionnaires. Also, the study did not compare the validity of the 2-question screen or PHQ-9 with that of other postpartum depression screens, such as the Edinburgh Postnatal Depression Scale.
This study’s findings contribute important information on scientifically based methods for identifying postpartum depression—the most common serious obstetric complication. If confirmed by additional studies, our validity test results suggest that the highly sensitive 2-question screen and highly specific PHQ-9 perform well together in a 2-stage screening procedure, whereby women initially complete the 2-question screen, and those whose results are positive complete the PHQ-9. Women who have positive findings on the PHQ-9 would be advised to see their clinician for further evaluation and treatment.
Footnotes
-
Conflicts of interest: none reported
-
Funding support: This study was funded by the National Institute of Mental Health (Dr Gjerdingen: R34 MH072925; Dr Crow: K02-MH65919; P30 DK50456).
- Received for publication April 7, 2008.
- Revision received July 3, 2008.
- Accepted for publication July 14, 2008.
- © 2009 Annals of Family Medicine, Inc.