Abstract
PURPOSE Findings are conflicting about the relationship between vitamin D levels and cardiovascular mortality. We wanted to determine the contribution of vitamin D levels to black-white disparities in cardiovascular mortality.
METHODS We examined the association of serum 25(OH)D levels with cardiovascular mortality and its contribution to elevated risk among blacks through a retrospective cohort using baseline data from the third National Health and Nutrition Examination Survey 1988–1994 and cause-specific mortality through 2001 using the National Death Index. Using piecewise Poisson regression models, we examined the risk of cardiovascular death (coronary heart disease, heart failure, and stroke) by sample 25(OH)D quartile, adjusting for cardiovascular risk factors, and compared models of adjusted race-related cardiovascular mortality with and without further adjustment for 25(OH)D levels.
RESULTS Participants with 25(OH)D levels in the lowest quartile (mean = 13.9 ng/mL) compared with those in the 3 higher quartiles (mean = 21.6, 28.4, and 41.6 ng/mL) had higher adjusted risk of cardiovascular death (incident rate ratio [IRR] = 1.40; 95% confidence interval [CI], 1.16–1.70). The higher age- and sex-adjusted cardiovascular mortality observed in blacks vs whites (IRR = 1.38; 95% CI, 1.13–1.70) was attenuated (IRR = 1.14; 95% CI, 0.91–1.44) by adjustment for 25(OH)D levels and fully eliminated with further adjustment for income (IRR=1.01; 95% CI, 0.82–1.24).
CONCLUSIONS Low serum levels of 25(OH)D are associated with increased cardiovascular mortality in a nationally representative US sample. Black-white differences in 25(OH)D levels may contribute to excess cardiovascular mortality in blacks. Interventional trials among persons with low vitamin D levels are needed to determine whether oral supplementation improves cardiovascular outcomes.
INTRODUCTION
Evidence links low levels of vitamin D, particularly levels of 25, hydroxyvitamin D, or 25[OH]D, to cardiovascular disease.1,2 Low serum levels (and/or low dietary and supplement intake) have been linked to cardiovascular risk factors that include obesity,3,4 hypertension,3,5,6 diabetes,3,7 peripheral arterial disease,8 chronic renal disease,9 and heart disease. Studies have also linked low levels to incident cardiovascular disease,10 including myocardial infarction.11
Low levels of vitamin D may alter the renin-angiotensin axis by direct suppression of renin gene expression12; affect vascular endothelium through smooth muscle proliferation,13 inflammation,12 and thrombosis14; alter calcium channel fluxes; and induce secondary hyperparathyrodism. Elevated parathyroid hormone has been linked to mortality in the elderly,15 possibly through vascular effects,16 although findings regarding an association with cardiovascular mortality have been mixed.17,18 These findings suggest pathways through which low levels of vitamin D may increase the risk of cardiovascular-related morbidity (including hypertension, diabetes, renal, heart and cerebrovascular disease) and subsequent cardiovascular mortality.
Relative to whites, blacks have higher rates of many cardiovascular risk factors and higher age- and sex-adjusted cardiovascular mortality.19 This higher risk is accounted for by traditional risk factors (many of which are more common among blacks) and by lower socioeconomic status (itself associated with traditional cardiovascular risk factors).20,21 Low-socioeconomic status is associated with greater chronic stress, poorer access to health care, and fewer resources for ameliorating behavioral risk factors. Blacks have significantly lower mean serum 25(OH)D levels,3,22 related both to biological (increased skin pigmentation reducing activation of oral vitamin D23) and social and behavioral (lower intake,24 less sun exposure25) factors. Thus, lower levels of 25(OH)D may contribute to black-white disparities in cardiovascular mortality acting through racial differences in both biological and social or behavioral factors that increase the risk of cardiovascular-related conditions and subsequent mortality.
We examined whether (1) serum 25(OH)D predicts subsequent cardiovascular mortality among a representative sample of US adults, and (2) differences in 25(OH)D levels contribute to black-white differences in age- and sex-adjusted cardiovascular mortality.
METHODS
Participants
We constructed a retrospective cohort using publicly available data from the nationally representative Third National Health and Nutrition Examination Survey (NHANES III) conducted from 1988–1994.26 Our sample was restricted to participants aged 18 years and older. Complete data for all variables (including vital status) were available on 15,363 (78.3%) persons, corresponding to 90.8% of the target population (weighted).
Vitamin D
Serum 25(OH)D was measured using a radioimmuno-assay kit (DiaSorin, Stillwater, Minnesota).27 Levels were categorized into quartiles based on the unweighted sample. Although 1,25-dihydroxyvitamin D is the biologically active form of vitamin D, serum 25(OH)D is regarded as the best indicator of vitamin D status in individuals without kidney disease.10
Race and Ethnicity
We assessed race and ethnicity based on self-reported race (white, black, or other) and self-reported ethnicity (Hispanic or not). We classified participants as white (non-Hispanic), black (non-Hispanic), Hispanic, and other.
Cardiovascular Deaths
Assessment of death continued from data collection until December 31, 2000, based on the NHANES III Linked Mortality file, using International Classification of Diseases, 10th Revision (ICD-10) 3-digit codes.28 Cardiovascular mortality was based on codes I11–I78 (excluding I33–I40 [carditis]). Follow-up was censored at the date of death for persons who died of other diseases and at December 31, 2000, for those not identified as deceased.
Other Measures
We also collected data on additional measures that affect cardiovascular health: physical activity based on metabolic equivalent tasks (METs), body mass index (BMI), smoking status, poverty level, total cholesterol, serum creatinine, systolic blood pressure, serum albumin, albuminuria, C-reactive protein (CRP), glomerular filtration rate (eGFR), albumin-creatinine ratio (ACR), fasting blood glucose, and self-reported health, diabetes, and cardiovascular health. Details of these and supplementary measures and analyses are described in the Supplemental Appendix, available at: http://www.annfammed.org/cgi/content/full/8/1/11/DC1.
Statistical Analyses
Analyses were conducted with Stata (version 10.0, StataCorp, College Station, Texas), adjusting for the complex survey design of NHANES III to yield appropriate standard errors and population parameter estimates. We implemented Cox semiparametric proportional hazards survival analyses and compared these results with a proportional hazards parametric regression model of age-at-event failure time data specified as a log-linear model.29 This latter model was implemented by applying a piecewise Poisson regression procedure, using person years as the unit of analysis. That is, each subject contributed an observation for each full or partial year of follow-up. Instead of the adjusted hazard ratios (HRs) available from Cox regression, the Poisson regression yields adjusted incident rate ratios (IRRs); IRRs were quite similar to the HRs. An advantage of the parametric Poisson regression model is that, unlike the semiparametric Cox model, it allows formal statistical comparison of parameter estimates among nested models.30 We tested the proportionality assumption graphically and numerically and found no concerns. We report the Poisson model results. Cox model results are available on request.
The analysis examining the adjusted relationship between serum 25(OH)D and cardiovascular mortality controlled for age, log (age) (to adjust for the exponential relationship between age and mortality), sex, interview month (a series of dummy variables), region (a series of dummy variables), race and ethnicity (white, black, Hispanic, and other), household income using percentage of federal poverty level (<100%, 100%–149%, 150%–199%, 200%–299%, ≥300%), smoking status (current or not), physical inactivity (METs <3.5/mo), self-rated health (excellent, very good, good, fair, poor), self-reported diabetes (based on self-report, a fasting glucose >126 mg/dL, or glycohemoglobin >6.0%), self-reported baseline cardiovascular disease, BMI category (<20, 20–25, 25.1–29.9, ≥30 kg/m2), systolic blood pressure (<120, 120–139, ≥140 mm Hg), eGFR (mL/min per 1.73 m2), total cholesterol (<200, 200–239, 240–279, ≥280 mg/dL), serum albumin (mg/dL), CRP, urinary ACR, and serum 25(OH)D (as sample quartiles with cut points of <18, 18–24.9, 25–31.9, and ≥32 ng/mL* to examine possible threshold effects).
To examine the possible contribution of serum 25(OH)D to black-white differences in cardiovascular mortality, we used a nested modeling approach, examining 4 models. Because many of the covariates included above may themselves be in the pathway that connects race, vitamin D, and cardiovascular mortality (as noted in the introduction), we conducted an analysis that included only those exogenous variables unlikely to mediate the effects of 25(OH)D levels on cardiovascular mortality (age, log [age], sex, region, and month of examination). We compared that model to a model that also included serum 25(OH)D (to derive an upper estimate of the potential mediating effect of 25[OH]D), another that included percentage of poverty (to derive an upper estimate of the potential mediating effect of income), and finally, one with both 25(OH)D and poverty (to estimate the separate mediating effects of 25[OH]D and income on the relationship between race and cardiovascular mortality). Comparisons among models were conducted using the method of Clogg et al.30 Confidence intervals around percentage of mediation effect were derived using Fieller’s method.31
RESULTS
The mean serum 25(OH)D level in the sample was 29.5 ng/mL. Table 1⇓ displays the distribution of baseline characteristics by sample quartile of 25(OH)D levels. Sixteen percent of the target population had 25(OH)D levels in the lowest sample quartile. Higher plasma 25(OH)D levels were associated with younger age, being male or white, higher income, lower blood pressure, not smoking, a lower BMI, a lower total cholesterol or serum albumin, higher eGFR, lower ACR, lower CRP, greater physical activity, better self-rated health, less diabetes, and less baseline cardiovascular morbidity.
Distribution of Characteristics (Means, Proportions) by Quartiles of Plasma 25(OH)D
There were 933 (43.7% of total mortality, population weighted) cardiovascular deaths among those with no missing data in 138,549 person-years of follow-up. Of the cardiovascular deaths, 25% were due to myocardial infarctions, 44% were due to ischemic and other heart disease, and 17% were due to stroke.
The adjusted association of risk factors with cardiovascular death is summarized in Table 2⇓. Cardiovascular death was independently associated with older age, male sex, non-Hispanic ethnicity, higher blood pressure, smoking, physical inactivity, being underweight (BMI <20.0 kg/m2), higher total cholesterol, higher CRP, lower eGFR, higher ACR, poorer self-rated health, and baseline diabetes and cardiovascular morbidity.
Adjusted Incident Rate Ratio (IRR) For Death From Cardiovascular Disease During Follow-Up Interval
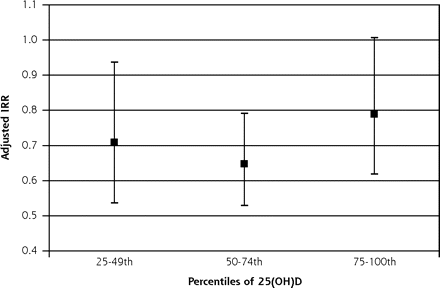
Higher 25(OH)D levels (relative to the lowest quartile) were associated with lower adjusted mortality (Figure 1⇓). There appeared to be a threshold effect with little reduction in cardiovascular deaths above the 25th percentile. Cardiovascular mortality in the highest 25(OH)D quartile was higher than that in the 2 middle quartiles, but differences among the higher 3 quartiles were not statistically significant (adjusted Wald test, F2,48=1.37, P=.26). The adjusted cardiovascular mortality risk for the lowest compared with the higher three 25(OH)D quartiles was 40% higher (IRR 1.40 (95% confidence interval [CI], 1.16–1.69, P = .001). An analysis excluding those with baseline cardiovascular-related morbidity (diabetes, cardiovascular disease, and eGFR <60 mL/min per 1.73 m2) revealed an adjusted IRR for low 25(OH)D of 1.97 (95% CI, 1.40–2.78, P<.001).
Adjusted incidence rate ratio (IRR) for quartiles of plasma 25(OH)D levels with lowest quartile as reference (IRR = 1).
25(OH)D = 25, hydroxyvitamin D.
We addressed our second aim, examining the relationship between race and cardiovascular mortality, and the potential mediating effect of 25(OH)D in a series of nested models (Table 3⇓). In the model adjusting only for exogenous variables (ie, those more clearly outside the causal pathway between 25(OH)D and cardiovascular mortality): age, log (age), sex, month, and region, blacks showed significantly higher cardiovascular mortality than whites (Table 3⇓, model 1). When 25(OH)D (Table 3⇓, model 2) was added, there was a significant reduction in the risk associated with black race (F1,49 = 24.9 (), P <.001). The proportional excess risk reduction for race ([IRR(BlackM1)–IRR(BlackM2)]/[IRR(BlackM1)–1]) between model 1 and 2 was 63% (95% CI, 35%–100%).
Incident Rate Ratios (IRR) For Death From Cardiovascular Disease During Follow-up Interval With Selected Adjustment
When income was added to the model (Table 3⇑, model 3), there was a significant reduction in the risk associated with black race (between the exogenous variable only model 1 and model 3, including income, F=14.7; P <.001). When both income and 25(OH)D were added to the exogenous variables (Table 3⇑, model 4), the point estimate for race was further reduced, this time to 1.0. The reductions in the IRR for race were significant between model 2 and 4 (F=11.2; P=.002) and between model 3 and 4 (F=28.9; P <.001), indicating that the effects of income and 25(OH)D were additive.
DISCUSSION
Our findings are notable in 2 respects. First, consistent with prior research, we observed an association between baseline low serum 25(OH)D levels and subsequent increased cardiovascular mortality. This association appeared to be partly mediated though cardiovascular-related conditions (hypertension, heart failure, myocardial infarction, stroke, kidney disease, and diabetes), but it was also observed when controlling for multiple existing cardiovascular risk factors, including many potential mediators. Second, we observed that low 25(OH)D levels substantially accounted for the higher age- and sex-adjusted cardiovascular mortality among blacks. We address both these findings in the context of the existing literature and study limitations.
In this nationally representative US sample, participants in the bottom sample quartile for serum 25(OH)D had 40% adjusted higher cardiovascular risk for death than those with higher levels. There appeared to be a threshold effect, with no evidence of further effects above the lowest quartile. In the supplemental analysis (shown in the Supplemental Appendix), lower vitamin D intake was associated with higher cardiovascular mortality; this association was no longer significant when 25(OH)D was included. These results add to existing evidence suggesting that low 25(OH)D levels may be an independent, potentially modifiable, cardiovascular risk factor.3–11,17
The finding that the adverse cardiovascular effect of a low 25(OH)D level was increased in the analysis excluding those with cardiovascular-related morbidity is consistent with the notion that the full analysis is overadjusted. That is, if 25(OH)D levels are causally related to the onset of cardiovascular-related morbidity, then those with cardiovascular-related morbidity at baseline presumably developed those conditions, in part, because of low 25(OH)D levels. Thus, including those participants in the analysis and adjusting for those conditions result in partially adjusting for cardiovascular effects of 25(OH)D that had already occurred at baseline. In contrast, the analysis excluding those with baseline cardiovascular-related morbidity likely reflects a less-overadjusted estimate of the effects of 25(OH)D on cardiovascular mortality.
Prospective studies suggest that low levels of vitamin D precede the development of cardiovascular-related conditions, such as diabetes and hypertension.6,32 Vitamin D may affect vascular endothelium directly through the renin-angiotensin axis12; through effects on vascular smooth muscle, including cell proliferation,13 inflammation,12 and thrombosis14; and through effects on hyperparathyroid hormone.15,16 We did not assess these factors directly, although we found an inverse relationship between 25(OH)D levels and CRP.
Our second notable finding pertains to the potential contribution of vitamin D to racial differences in age- and sex-adjusted cardiovascular mortality. Controlling for the large differences by race in 25(OH)D levels yielded a significant attenuation in the association of race with cardiovascular mortality. In the model that included only exogenous variables (age, sex, month, and region), blacks had a 38% higher rate of cardiovascular mortality than whites, comparable to recently reported racial disparity in cardiovascular mortality.33 Adjustment for 25(OH)D levels reduced this excess risk by approximately 60%. Inclusion of both 25(OH)D and poverty reduced the excess risk to 1.0, suggesting that low 25(OH)D levels and poverty exert separate, additive effects on black cardiovascular mortality.
These findings are consistent with the notion that higher cardiovascular risk for blacks is partly related to lower levels of 25(OH)D. Consistent with prior findings,34 race was strongly associated with low 25(OH)D levels. Such differences in levels by race have been reported in infancy.35,36 Lower levels among blacks potentially reflect decreased sun exposure,25 decreased sun absorption because of higher dermal melanin,23 and lower vitamin D intake.24 Supplements higher than those currently recommended would be needed to substantially increase levels among those in the lowest quartile.37,38
In an earlier study, Melamed et al observed an association between low 25(OH)D levels and all-cause mortality, but no significant effect on cardiovascular mortality.18 Using their analytic approach, we were able to produce comparable results. Two key differences between their and our analyses explain the discrepant results. Most importantly, they used the highest quartile of 25(OH)D for their reference group. As noted by Melamed et al and observed here (Table 2⇑ and Figure 1⇑), those in the highest quartile had an adjusted mortality rate slightly (but not significantly) higher than the middle two quartiles, and lower (but not significantly) than the bottom quartile; thus, their use of this referent masked significant differences between the lowest and other quartiles. Second, their analysis did not adjust for health status, which exhibits strong independent relationships with both 25(OH)D (Table 1⇑) and mortality (Table 2⇑).
There are several sources of potential residual confounding in our analysis. First, low 25(OH)D levels may represent a marker for poor health (inadequately controlled in this study) or poor health may result in reduced sun exposure and consequent lower 25(OH)D levels. Second, measurement error in recorded variables, changes in measures after the single baseline assessment, or unmeasured risk factors could confound the findings. Differences in risk associated with low 25(OH)D levels, however, were modest between the model including only exogenous variables (IRR = 1.73) and the potentially overadjusted model including possible intervening factors (IRR = 1.40). The modest changes between a model with multiple measured potential confounders and one with only exogenous variables suggest that unmeasured confounders may not fully account for the significant effect associated with low 25(OH)D levels.
Besides unmeasured confounding factors and the challenge of disentangling confounding from mediating factors, the other key limitation of this study is potential misclassification of cause of death. Death certificate data coding corresponds reasonably well with hospital data,39 with overestimation for myocardial infarction.40 The National Center for Health Statistics has perturbed data in the NHANES-NDI file to enhance confidentiality, but direct comparisons with restricted data show similar results.41 Most important, errors in coding of deaths or perturbations of the data would bias results toward the null.
To date, there are limited data from randomized controlled trials (RCTs) regarding the impact of vitamin D supplementation on cardiovascular disease. A meta-analysis of RCTs of vitamin D supplementation for other purposes, such as improvement in bone density and reduction in fractures, showed a reduction in all-cause mortality.42 A small, short-term RCT involving cholecalciferol (vitamin D3) plus calcium vs calcium alone among elderly women with low 25(OH)D levels showed modest reductions in systolic blood pressure in those receiving the combined intervention.43 Three RCTs of a vitamin D analogue, paricalcitol, yielded reductions in proteinuria among patients with chronic kidney disease.44 Some have suggested that statins represent analogues of vitamin D.45 Thus, there is emerging evidence for a potential causal and remediable relationship between low vitamin D levels and cardiovascular-related disease.
These findings highlight the need for RCTs to assess the impact of vitamin D supplementation on the development of cardiovascular-related conditions and mortality. Such studies should include sufficient numbers of blacks to assess the potential for supplementation among those with low levels to improve cardiovascular outcomes and reduce disparities. In the absence of such trials, the current observational findings are suggestive only. Previous observational studies showed a relationship between other vitamins (eg, vitamin E and beta-carotene) and cardiovascular mortality46–48 that were not borne out by subsequent RCTs.49,50
In conclusion, low 25(OH)D levels independently predict cardiovascular mortality in a national US sample, with an apparent threshold effect around the 25th percentile. High prevalence of low 25(OH)D levels among blacks appears to contribute to differences in black-white age- and sex- adjusted cardiovascular mortality. RCTs of vitamin D supplementation in those with low 25(OH)D levels are needed to determine whether optimization of these levels improves outcomes from cardiovascular mortality, particularly among blacks, who bear a disproportionate burden of cardiovascular disease.
Footnotes
-
↵* To convert to SI units (nmol/L), multiply by 2.496.
-
Conflicts of interest: none reported
-
Funding support: The study was supported by funding through The National Heart Lung and Blood Institute (1R01 HL081066-01A2).
- Received for publication November 12, 2008.
- Revision received February 10, 2009.
- Accepted for publication March 16, 2009.
- © 2010 Annals of Family Medicine, Inc.