Abstract
PURPOSE HIV pre-exposure prophylaxis (PrEP) may increase rates of bacterial sexually transmitted infections (STIs) among gay, bisexual, and other men who have sex with men (GBM) through risk compensation (eg, an increase in condomless sex or number of partners); however, longitudinal studies exploring the time-dependent nature of PrEP uptake and bacterial STIs are limited. We used marginal structural models to estimate the effect of PrEP uptake on STI incidence.
METHODS We analyzed data from the iCruise study, an online longitudinal study of 535 Ontarian GBM from July 2017 to April 2018, to estimate the effects of PrEP uptake on incidence of self-reported bacterial STIs (chlamydia, gonorrhea, and syphilis) collected with 12 weekly diaries. The incidence rate was calculated as the number of infections per 100 person-months, with evaluation of the STIs overall and individually. We used marginal structural models to account for time-varying confounding and quantitative bias analysis to evaluate the sensitivity of estimates to nondifferential outcome misclassification.
RESULTS Participating GBM were followed up for a total of 1,623.5 person-months. Overall, 70 participants (13.1%) took PrEP during the study period. Relative to no uptake, PrEP uptake was associated with an increased incidence rate of gonorrhea (incidence rate ratio = 4.00; 95% CI, 1.67-9.58), but not of chlamydia or syphilis, and not of any bacterial STI overall. Accounting for misclassification, the median incidence rate ratio for gonorrhea was 2.36 (95% simulation interval, 1.08-5.06).
CONCLUSIONS We observed an increased incidence rate of gonorrhea associated with PrEP uptake among Ontarian GBM that was robust to misclassification. Although our findings support current guidelines for integrating gonorrhea screening with PrEP services, additional research should consider the long-term impact of PrEP among this population.
Annals Early Access article
- bacterial sexually transmitted infections
- STIs
- pre-exposure prophylaxis
- PrEP
- HIV
- gay, bisexual, and men who have sex with men
- sexual and gender minorities
- health risk behaviors
- prevention
- marginal structural models
- quantitative bias analysis
- risk compensation
- vulnerable populations
INTRODUCTION
Bacterial sexually transmitted infections (STIs), including chlamydia, gonorrhea, and syphilis, have increased since 2010, with cases of gonorrhea and syphilis commonly reported among men in Ontario, Canada.1 In 2014, an estimated 40% of gonorrhea cases and 85% of syphilis cases occurred among gay, bisexual, and other men who have sex with men (GBM).2 This disproportionate burden is of concern as untreated bacterial STIs can cause serious sequelae such as epididymitis, orchitis, prostatitis, urethral strictures, and infertility.3,4 Various factors contribute to the increasing incidence of bacterial STIs including expanded extragenital testing,5 substance use,6 and a reduction in condom use, partly attributed to the availability of HIV pre-exposure prophylaxis (PrEP). These associations have raised concerns about sexual risk compensation (eg, an increase in condomless sex or number of partners), given the concurrent increase in PrEP use and bacterial STI diagnosis among GBM.7-11
It is hypothesized that PrEP use could reduce condom use and increase the risk of bacterial STI transmission among GBM.10,11 Research on the associations among PrEP use, sexual behavior, and bacterial STIs in this population has netted inconsistent findings, however.12,13 Some studies have found associations between PrEP use, condomless anal sex, and bacterial STIs,14-18 whereas others have not.19,20 These disparate findings may reflect a methodologic challenge, that is, the bidirectional relationships of PrEP uptake, sexual behavior, and bacterial STI incidence. For example, a history of bacterial STI is often an indicator for initiating PrEP21 because it may reflect sexual networks with higher bacterial STI prevalence and greater risk of HIV exposure, or an indicator for increased testing as part of routine PrEP care (Supplemental Figure 1). Moreover, condomless anal sex may both confound future PrEP uptake and bacterial STI incidence and mediate past exposure–outcome associations. Given this complexity, traditional regression analysis approaches may not suffice, necessitating the use of marginal structural models to account for the time-varying effect of PrEP uptake on bacterial STI incidence.
To better understand this complex relationship, we considered whether the trajectory of PrEP uptake over 3 months functions as a determinant of bacterial STI incidence using observational data from an online study of Ontarian GBM.22 We hypothesized that PrEP uptake would affect bacterial STI incidence, whereby those taking PrEP would have a higher incidence rate than those not taking PrEP after accounting for baseline and time-varying covariates.
METHODS
Study Design and Participants
The iCruise study is a longitudinal, online, mixed-methods study designed to assess the sexual health-seeking behaviors of GBM in Ontario, Canada.23 Participants were purposively sampled with respect to 8 GBM subgroups (aged >50 years old, living with HIV, immigrants, ethnoracial minority, transgender, rural resident, straight-identified, and those encountered online by outreach from sexual-health workers) between July 2017 to April 2018 using sociosexual networking sites, mobile apps, and a community-based e-mail listserv. Eligible participants were men who were aged 14 years or older; identified as gay, bisexual, 2-spirit, straight, queer, or questioning; had sex with another man in the past 12 months; and lived in, worked in, or visited Ontario at least 4 times in the past year.
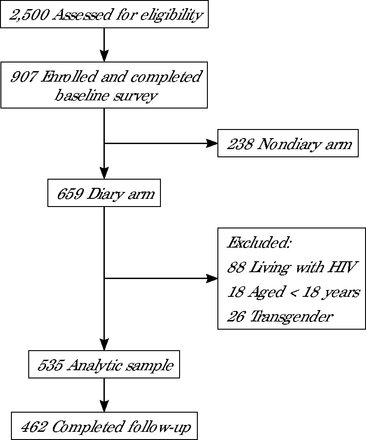
A total of 907 eligible men enrolled and 659 were randomly selected to participate in a weekly diary survey for up to 12 weeks after baseline (Figure 1). During follow-up, participants were asked to complete at least 10 of 12 weekly electronic diary surveys; those who missed 4 or more consecutive diary surveys were considered lost to follow-up. For our analysis, we further restricted the sample to HIV-negative, cisgender GBM aged 18 years or older.
Recruitment and retention in the iCruise study.
Note: Participants were defined as not completing follow-up if they missed ≥4 consecutive diary surveys.
Participants provided informed consent and received compensation of up to $80 for completing the surveys. Ethical approval was granted by the Research Ethics Board of the University of Toronto.
Measures
Outcome
During each diary survey, participants were asked: “Have you been told that you have an STI and/or were you treated for an STI (not including HIV) in the past 7 days?” If they answered yes, participants were then asked to report the specific STI. We defined the incidence rate of any bacterial STI as the number of chlamydia, gonorrhea, and syphilis infections per 100 person-months. These STI were also examined separately.
Exposure
PrEP uptake was based on the questions, “Are you currently taking PrEP?” (yes/no) asked at baseline and “In the past week, did you take PrEP (pre-exposure prophylaxis?” (yes/no) asked during diary follow-up.
Person-Time
We used person-months in analyses because of the diary study design and the relatively short follow-up period of the iCruise study. Follow-up commenced on enrollment.24 We assigned participants to either the PrEP uptake group or PrEP nonuptake group based on their self-report of current PrEP use. Those in the PrEP uptake group (based on a “yes” response to the baseline and/or follow-up exposure question) contributed person-time to that group until discontinuation, loss to follow-up, or study completion. We defined discontinuation so that it aligned to a typical 90-day PrEP prescription,21 meaning that participants had to stop reporting PrEP uptake for 10 consecutive weeks to be coded as “discontinued.” In the nonuptake group, participants contributed person-time to that group until they reported initiating PrEP or were lost to follow-up, or the study ended.
Covariates
Guided by a directed acyclic graph (Supplemental Figure 1), we considered in analyses a variety of baseline covariates (age, employment, income, access to primary care clinician, alcohol and drug use, and lifetime bacterial STI diagnosis) and time-varying covariates (number of anal sex partners, condomless anal sex, bacterial STI screening, and prior PrEP uptake during follow-up). We considered ethnoracial identity and sexual orientation as descriptor variables only, not as model covariates.
Statistical Analyses
We computed statistics for key sociodemographic and behavioral characteristics of iCruise participants overall and by reported PrEP uptake. Using bivariate analyses, we examined the differences in baseline characteristics by PrEP uptake. Hypothesis testing was conducted using t tests for continuous variables and Pearson χ2 tests or Fisher exact tests for categorical variables. Moreover, we evaluated number of anal sex partners and percentage of individuals reporting condomless anal sex during the period before and the period after the initial change in PrEP initiation over follow-up.
To adjust for differences between GBM with and without PrEP uptake, we fitted a series of models. Model 1 (unadjusted) ignored the time-varying nature of PrEP uptake and modeled the unadjusted association of PrEP uptake and incidence rate of bacterial STIs using generalized estimating equation (GEE) Poisson models with clustered robust standard errors. Person-months were included as an offset term. In model 2 (adjusted), we performed regression adjustment for baseline covariates; however, this approach would produce biased estimates in the presence of time-varying confounding.25 In model 3 (marginal structural), we therefore incorporated inverse probability of exposure weights to account for time-varying confounding and estimate the marginal effects of PrEP uptake on bacterial STIs. To construct these weights, we calculated the stabilized inverse probability of exposure weights for each follow-up visit. These weights are generated based on propensity scores using logistic regression analysis. As this analysis defaults to an analysis of cases having complete data, we used multiple imputation by chained equations, assuming that covariates were missing at random. Before fitting the weighted GEE Poisson model, we examined the distribution of nonstabilized and stabilized weights between groups with and without PrEP uptake during follow-up using boxplots (Supplemental Figure 2 and Supplemental Figure 3).
To adjust for potential selection bias due to differential loss to follow-up, we used a similar procedure to estimate the inverse probability of attrition weights using logistic regression analysis. We compared baseline covariates between those who remained in the study and those who failed to complete follow-up (ie, missed ≥4 diary surveys consecutively) to identify factors that may influence attrition. Final weights for each participant were generated by multiplying exposure weights across all follow-up periods and then multiplying those results by the attrition weights.
Sensitivity Analyses
We performed 3 sensitivity analyses. First, we accounted for the potential incubation period of bacterial STIs (2 to 21 days)3 by lagging PrEP uptake from 1 to 3 weeks. Second, we calculated the E-value and its lower bound for weighted models to assess the impact of unmeasured confounding.26 Third, considering underreporting due to asymptomatic infections3 or social desirability bias, we conducted quantitative bias analysis, assuming nondifferential outcome misclassification of bacterial STI incidence.27,28 Additional details regarding the development of the inverse probability of weights, quantitative bias analysis, characteristics of men who were lost to follow-up (Supplemental Table 1), and input parameters for the bias analysis (Supplemental Table 2 and Supplemental Table 3) are presented in the Supplemental Appendix. All analyses were conducted in R studio version 4.129 using the geepack30 and ipw31 package (R Foundation for Statistical Computing).
RESULTS
Overall, 535 participants in the iCruise study were included in our analytic sample, most of whom were gay and White (Table 1). The mean (SD) age at baseline was 34.0 (12.8) years and about one-half of participants (46.7%) had had sex partners in the past 3 months, with 18.3% and 11.1% having had more than 5 anal and oral sex partners, respectively. Nearly one-fifth (18.1%) had engaged in condomless sex in the past 3 months. The large majority (85.2%) had access to a primary care clinician. Past STI testing (36.1%) and lifetime STI diagnosis (45.2%) were fairly common. Participants reported an average of 3.4 anal sex partners during the week before initiating PrEP and 1.2 anal sex partners after PrEP initiation. The prevalence of condomless anal sex was similar during the week before (57.1%) and the week after (54.3%) PrEP initiation.
Characteristics of Included Men, Overall and by PrEP Uptake
During follow-up, 86.4% of participants remained in the study. Participants were followed up for a median of 3.3 months (interquartile range, 2.9-3.4). During 1,623.5 person-months (135.3 person-years) of follow-up, 13.1% of participants took PrEP (Table 2) and 1.1% discontinued PrEP. In the cohort overall, the crude incidence rates per 100 person-months were 5.5 (95% CI, 4.5-6.8) for any bacterial STI, 2.5 (95% CI, 1.8-3.4) for chlamydia, 1.8 (95% CI, 1.2-2.6) for gonorrhea, and 1.2 (95% CI, 0.8-1.9) for syphilis.
Incidence of Bacterial STIs During Follow-up, Overall and by PrEP Uptake
After accounting for confounding and selection bias using the inverse probability of exposure and attrition weights, the incidence rate of any bacterial STI was higher among iCruise participants who took PrEP compared with those who did not (incidence rate ratio [IRR] = 1.86; 95% CI, 0.86-4.04; Table 3); however, this estimate was imprecise and not statistically significant. The effect of PrEP uptake on the incidence rate varied across the bacterial STIs, with an adjusted IRR of 1.22 (95% CI, 0.53-2.81) for chlamydia, 4.00 (95% CI, 1.67-9.58) for gonorrhea, and 1.05 (95% CI, 0.28-3.92) for syphilis.
Estimates of the Association Between PrEP Uptake and Incidence of Bacterial STIs
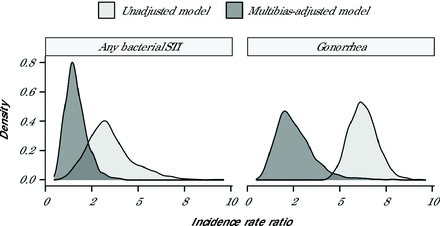
Sensitivity analyses indicated that associations between PrEP uptake and bacterial STI incidence were robust to varying assumptions about the timing of PrEP uptake (Supplemental Figure 4). For gonorrhea, the E-value was about 7.5, suggesting an unmeasured confounder would need to be associated with both PrEP uptake and bacterial STI incidence with an IRR of at least 7.5 beyond measured covariates to explain away the results (Table 3). Quantitative bias analysis indicated a median IRR of 1.55 (95% simulation interval, 0.76-3.01) for any bacterial STI and 2.36 (95% simulation interval, 1.08-5.06) for gonorrhea in PrEP users compared with nonusers (Figure 2). Thus, nondifferential outcome misclassification biased the results away from the null with an artificially inflated association between PrEP uptake and gonorrhea incidence.
Unadjusted and multibias-adjusted density of incidence rate ratios among GBM by bacterial sexually transmitted infection.
GBM = gay, bisexual, and other men who have sex with men; STI = sexually transmitted infection.
Note: Incidence rate ratios compare GBM with vs without uptake of pre-exposure prophylaxis. Unadjusted density is based on 1,000 bootstrapped replications of the unadjusted model. Multibias-adjusted density is based on the simulated distribution of the true incidence rate while accounting for confounding and losses to follow-up using inverse probability of exposure and attrition weights, respectively. Results are not shown for chlamydia and syphilis because of the null findings in unadjusted models. Furthermore, because of the low incidence rate for syphilis, simulated incidence rate ratios in the multibias-adjusted model resulted in unstable estimation for that STI.
DISCUSSION
In this online longitudinal study, PrEP uptake was associated with a higher incidence rate of gonorrhea among GBM after adjusting for confounding, attrition, and nondifferential outcome misclassification. We did not find sufficient evidence to indicate higher incidence rates of chlamydia and syphilis among men using PrEP during follow-up. The magnitude of risk for gonorrhea among those taking PrEP suggests a need to focus on ancillary services (eg, STI testing) that can support the sexual health needs of GBM taking PrEP and potentially novel interventions for preventing transmission such as doxycycline post-exposure prophylaxis (doxy-PEP).32,33 For example, there is growing recognition that comprehensive STI prevention requires the presence of primary care,34 and in many cases, family medicine practitioners are well positioned to serve as the main contact point for GBM, offering valuable information about PrEP, prescribing these medications, and supporting ancillary services to ensure long-term retention in care.
Our findings on PrEP uptake and risk compensation are consistent with some previous reports from observational studies in high-resource settings that showed an association between PrEP uptake and bacterial STIs.13,16-18 For instance, in a longitudinal study using propensity score–matched historical controls, investigators found that the incidence rate of bacterial STIs over time increased by 2.4-fold in participants who initiated PrEP at a sexually transmitted disease clinic in the United States.17 The investigators also reported associations between PrEP initiation and each bacterial pathogen, including chlamydia, gonorrhea, and syphilis. Similar results were also reported among Australian GBM and indicated a higher incidence rate of bacterial STIs after PrEP initiation among those who did not have any prior experiences with PrEP.18
We did not have sufficient evidence, however, that PrEP uptake was associated with an increased incidence rate of any bacterial STI, chlamydia, or syphilis. This difference may reflect a higher prevalence of gonorrhea within sexual networks in Ontario during the time in which our data were collected. According to the 2017 European Men-who-have-sex-with-men Internet Survey, the prevalence of gonorrhea (24.6%) was higher than that of chlamydia (22.4%) and syphilis (13.7%) among GBM in Ontario that year,35 with data suggesting rising rates in Canada during the past few years.7-9 Moreover, differences in the transmission probability, incubation period, and intensity of symptoms of gonorrhea vs other bacterial STIs3,36,37 may also contribute to the increased reporting of gonorrhea in the shorter follow-up period. These data indicate that the potential decrease in condom use by iCruise participants while using PrEP may further contribute to the proliferation of gonorrhea transmission among Ontarian GBM.
Although more research with longer follow-up is needed to understand the long-term impact of PrEP uptake on gonorrhea, our findings support calls to further integrate STI risk-reduction counseling and testing services within PrEP programs,21,38 ensuring that PrEP adopters understand its benefits and limitations, and have access to care that addresses their sexual health needs. Furthermore, recent evidence from the US DoxyPEP trial shows a 65% (95% CI, 54%-73%) reduction in bacterial STI incidence over a 3-month period33 and similar effectiveness for people living with HIV, whether taking PrEP or not.39 Restricting doxy-PEP to people taking HIV PrEP therefore may not be warranted. Our results provide some support for this recommendation for more lenient prescribing as transmission rates for any bacterial STI, chlamydia, and syphilis were similar regardless of PrEP uptake. Ongoing surveillance is essential, however, to address tetracycline resistance concerns in gonorrhea.40
Our study has several limitations. It relied on a shorter follow-up period with data collected from 2017 to 2018, during which the landscape of PrEP uptake changed. We made important assumptions to obtain valid estimates, including consistency, exchangeability, positivity, and no model misspecification.25 We could include only confounders available in the iCruise study data, however, potentially omitting other important ones. We did not differentiate between partner role (insertive vs receptive) when including condomless anal sex as a time-varying covariate in our models, possibly contributing to residual confounding. Although we assumed nondifferential misclassification, it is possible that differential misclassification may be present as participants’ PrEP status may affect their probability of receiving STI testing and subsequent diagnosis. We were unable to evaluate this potential bias given the lack of external validation data within PrEP uptake strata. Furthermore, if validation data do not accurately represent the iCruise study population, this may further bias the findings. Increasing the statistical uncertainty of the sensitivity and specificity parameters may help mitigate this limitation. Although our analysis accounted for outcome misclassification, there is also the potential of exposure misclassification of reported PrEP uptake due to social desirability bias. The use of inverse probability of attrition weights strengthens the validity of the estimates, but also relies on the same assumptions as exposure weights, which might not fully adjust for selection bias. Finally, recruitment was primarily through sex-seeking social media sites, which introduces potential self-selection bias.
CONCLUSIONS
Our findings elucidate the early impact of PrEP uptake on reported bacterial STI incidence among Ontarian GBM. As bacterial STI rates rise, understanding the etiologic mechanisms will be crucial. Our findings, however, do not detract from PrEP’s role in HIV prevention. Rather, they underscore the importance of investing in STI testing, risk reduction, secondary prevention measures such as doxy-PEP, surveillance, and partner management—alongside PrEP—to reduce bacterial STI transmission effectively.
Footnotes
Conflicts of interest: authors report none.
Funding support: The iCruise study work was supported by the Canadian Foundation for AIDS Research (grant #026-009) and Canadian Institute of Health Research (grant #144830).
Disclaimer: The views expressed are solely those of the authors and do not necessarily represent official views of the authors’ affiliated institutions or funders.
Previous presentations: A portion of this work was presented at the 2023 annual meeting of the Society of Epidemiologic Research Portland; June 13-16, 2023; Portland, Oregon.
- Received for publication September 25, 2023.
- Revision received June 6, 2024.
- Accepted for publication June 7, 2024.
- © 2024 Annals of Family Medicine, Inc.