Abstract
PURPOSE We studied the efficacy of osteopathic manual treatment (OMT) and ultrasound therapy (UST) for chronic low back pain.
METHODS A randomized, double-blind, sham-controlled, 2 × 2 factorial design was used to study OMT and UST for short-term relief of nonspecific chronic low back pain. The 455 patients were randomized to OMT (n = 230) or sham OMT (n = 225) main effects groups, and to UST (n = 233) or sham UST (n = 222) main effects groups. Six treatment sessions were provided over 8 weeks. Intention-to-treat analysis was performed to measure moderate and substantial improvements in low back pain at week 12 (30% or greater and 50% or greater pain reductions from baseline, respectively). Five secondary outcomes, safety, and treatment adherence were also assessed.
RESULTS There was no statistical interaction between OMT and UST. Patients receiving OMT were more likely than patients receiving sham OMT to achieve moderate (response ratio [RR] = 1.38; 95% CI, 1.16-1.64; P <.001) and substantial (RR = 1.41, 95% CI, 1.13-1.76; P = .002) improvements in low back pain at week 12. These improvements met the Cochrane Back Review Group criterion for a medium effect size. Back-specific functioning, general health, work disability specific to low back pain, safety outcomes, and treatment adherence did not differ between patients receiving OMT and sham OMT. Nevertheless, patients in the OMT group were more likely to be very satisfied with their back care throughout the study (P <.001). Patients receiving OMT used prescription drugs for low back pain less frequently during the 12 weeks than did patients in the sham OMT group (use ratio = 0.66, 95% CI, 0.43-1.00; P = .048). Ultrasound therapy was not efficacious.
CONCLUSIONS The OMT regimen met or exceeded the Cochrane Back Review Group criterion for a medium effect size in relieving chronic low back pain. It was safe, parsimonious, and well accepted by patients.
- osteopathic manipulation
- ultrasonic therapy
- spinal manipulations
- spine
- low back pain
- chronic pain
- pain management
- complementary therapies
- manual therapies
INTRODUCTION
Low back pain is primarily responsible for more than 20 million ambulatory medical care visits1 and $100 billion in costs2 annually in the United States. When low back pain persists for 3 months, it is considered chronic and may cause progressive physical and psychological effects.3 Although practice guidelines recommend considering spinal manipulation for chronic or persistent low back pain,4,5 a Cochrane Collaboration review concluded that spinal manipulation is not more effective than sham interventions for short-term relief of chronic low back pain.6 The effectiveness of spinal manipulation remains controversial among family physicians.7 Osteopathic manual treatment (OMT) is delivered by osteopathic physicians in the United States, and by osteopaths in many other nations. No trial of OMT has conclusively found efficacy in relieving low back pain8–13 or achieved a status of low risk of bias.6 High-quality trials of ultrasound therapy (UST) are also needed to assess its efficacy compared with sham procedures.14 The OSTEOPAThic Health outcomes In Chronic low back pain (OSTEOPATHIC) Trial aims to fill these voids by studying OMT and UST for shortterm relief of nonspecific chronic low back pain.
METHODS
Design Overview
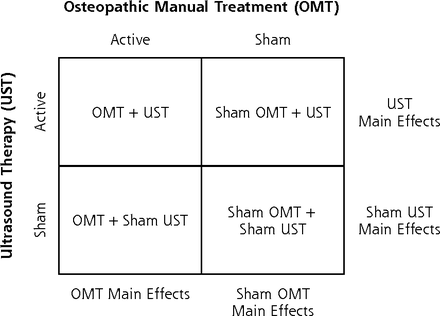
The OSTEOPATHIC Trial used a randomized, double-blind, sham-controlled, 2 × 2 factorial design to study OMT and UST.15 The study was designed to compare main effects (OMT vs sham OMT, and UST vs sham UST) (Figure 1). It was approved by the Institutional Review Board at the University of North Texas Health Science Center.
Allocation of patients to osteopathic manual treatment and ultrasound therapy interventions using a 2 × 2 factorial design.
Setting and Patients
We recruited patients in Dallas-Fort Worth, Texas, from August 2006 to September 2010 through newspaper advertisements, community agencies, and medical clinics, including those affiliated with the group practice of the University of North Texas Health Science Center, but excluding clinics that provided OMT specialty services. Adult (aged 21 to 69 years) nonpregnant individuals with low back pain for at least 3 months were telephonically screened for the following exclusion criteria: red flag conditions (cancer, spinal osteomyelitis, spinal fracture, herniated disc, ankylosing spondylitis, or cauda equina syndrome); low back surgery in the past year; workers’ compensation benefits in the past 3 months; ongoing litigation involving back problems; angina or congestive heart failure symptoms with minimal activity, history of a stroke, or transient ischemic attack in the past year; implanted biomedical devices (such as cardiac pacemakers or artificial joints); active bleeding or infection in the lower back, or other conditions impeding protocol implementation; use of corticosteroids in the past month; or use of manual treatment (OMT or manual therapies delivered by chiropractors or physical therapists) or UST in the past 3 months or more than 3 times in the past year. Candidates whose screening was successful by telephone received a clinical screening to exclude those with a high probability of lumbar radiculopathy, a specific cause of low back pain and a relative contraindication to OMT. Clinical screening involved testing for ankle dorsiflexion weakness, great toe extensor weakness, impaired ankle reflexes, loss of light touch sensation in the medial, dorsal, and lateral aspects of the foot, ipsilateral straight leg raising, and crossed straight leg raising.16
Randomization, Allocation Concealment, and Blinding
Patients were allocated to OMT + UST, OMT + sham UST, sham OMT + UST, or sham OMT + sham UST at the central randomization site based on a computer program that generated pseudorandom numbers. Patients were secondarily allocated to type of physician (faculty physician, predoctoral fellow, or resident) using stratified randomization. Assignments were then conveyed directly to the physicians using numbered, opaque sealed envelopes, which were subsequently placed in secured, segregated treatment files. Patients and outcome assessors remained unaware of group assignments at randomization.
Interventions
Treatments were scheduled at weeks 0, 1, 2, 4, 6, and 8 using 15 different physicians. We maintained the same physician at recurring treatment sessions for a given patient unless there was a scheduling conflict. Patients could self-initiate low back pain co-treatments, such as nonprescription drugs and complementary and alternative medicine therapies. Patients could also independently receive low back pain usual care (any co-treatments except OMT, other manual therapies, or UST) at any time from physicians not associated with the study. Co-treatments were documented at 4-week intervals throughout the study.
Active and Sham Osteopathic Manual Treatment
The OMT techniques were delivered after a standard diagnostic evaluation17 at each treatment session. The lumbosacral, iliac, and pubic regions were targeted using high-velocity, low-amplitude thrusts; moderate-velocity, moderate-amplitude thrusts; soft tissue stretching, kneading, and pressure; myofascial stretching and release; positional treatment of myofascial tender points; and patient’s isometric muscle activation against the physician’s unyielding and equal counter-force. Time permitting, optional techniques18 could be used if the physician judged 1 or more of the 6 designated techniques to be contraindicated or ineffective.
Sham OMT was aimed at the same anatomical regions as active OMT. Sham OMT involved hand contact, active and passive range of motion, and techniques that simulated OMT but that used such maneuvers as light touch, improper patient positioning, purposely misdirected movements, and diminished physician force. Similar methods achieved a robust placebo response13 when compared with other placebo treatments for pain.19 Our methods have been adopted by others to deliver sham manipulation.20
Active and Sham Ultrasound Therapy
The UST intervention was delivered after the OMT intervention, using the Sonicator 730 (Mettler Electronics Corp), with a 10 cm2 applicator at an intensity of 1.2 W/cm2 and frequency of 1 MHz in continuous mode. Conductivity gel was used to enhance absorption and produce deep muscle thermal effects.21 About 150 to 200 cm2 of the lower back were treated. Sham UST was delivered in the same manner at a subtherapeutic intensity (0.1 W/cm2).22
Treatment Fidelity and Adherence
Training for physicians delivering the treatment was conducted at regular intervals using strategies to enhance protocol implementation and treatment fidelity, including provision of sham treatments.23 A standard form was used at each treatment session to ensure consistency in delivery of active or sham OMT for 15 minutes, and of active or sham UST for 10 minutes. Both OMT and UST interventions were delivered by the same physician during a treatment session. Treatment adherence and reported pain levels were used as surrogate measures of patient blinding.
Outcomes
Primary Low Back Pain Outcomes
The current level of low back pain was measured before each treatment and at week 12 using a 100-mm visual analog scale. Primary outcomes were based on the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) consensus statement recommendations for moderate (30% or greater pain reduction) and substantial (50% or greater pain reduction) improvement.24 Such reductions are highly sensitive and specific in predicting global impression of change in chronic pain patients25 and provide tangible evidence for clinical applications.26
Secondary Outcomes
Patient-based secondary outcomes27 were measured at baseline and at weeks 4, 8, and 12, using the Roland-Morris Disability Questionnaire (RMDQ),28 Medical Outcomes Study Short Form-36 Health Survey general health scale (SF-36 GH),29 number of lost work days in the past 4 weeks because of low back pain, and satisfaction with back care on a 5-point Likert scale. We measured 8 low back pain co-treatments reported by patients as being self-initiated or received from independent, community-based clinicians.
Safety Monitoring
Blinded research personnel assessed patients for contraindications to continued participation or adverse events at each encounter. An independent safety officer reviewed all reported contraindications and adverse events to identify any serious adverse events, defined as deaths, life-threatening situations, hospitalizations, severe or permanent disability, or other important medical events. The safety officer also assessed causality of serious adverse events in relation to study interventions.
Sample Size
The planned sample size of 488 was designed for a statistical power of 82% or greater in testing OMT vs sham OMT main effects (standardized mean difference of 0.264 for OMT vs control treatments,30 corresponding to a between-group difference of 6.6 mm on a 100-mm visual analog scale, with a standard deviation of 25 mm31). In September 2010, under supervision of the Data and Safety Monitoring Board, an unplanned interim analysis was performed using visual analog scale change scores over 12 weeks that were measured through the provisional study end date of June 2010. This analysis was undertaken to determine whether the study could be terminated with 455 (93%) of the planned number of patients having enrolled in the study. Alternatively, additional sources of funding would have been required for further recruitment and study completion. The results of this analysis indicated that the change scores for OMT patients were significantly better than for sham OMT patients (P = .003). This finding crossed the O’Brien-Fleming stopping boundary (P = .005).32 Consequently, study recruitment was terminated.
Statistical Analysis
Low Back Pain Outcomes
We observed a bimodal distribution of visual analog scale change scores and corresponding marginal test for normality (P = .08). Consequently, we used the median and interquartile range (IQR) as descriptive measures, and the Mann-Whitney test and contingency table methods for analysis of low back pain outcomes. Responder analysis was used to assess treatment effects at week 12, with a focus on moderate and substantial improvements in low back pain.24 Response ratios (RRs) and 95% confidence intervals for active vs sham treatments were used to interpret treatment effects. Significant results were considered clinically relevant if they met the Cochrane Back Review Group criteria for medium (1.25 ≤RR ≤2) or large (RR >2) effect sizes.33
To test for statistical interaction between OMT and UST, we performed repeated measures analysis of variance on the ranked visual analog scale pain scores for each protocol visit, with the ranked baseline pain score as a covariate. Rothman’s T statistic 34 was used to test for statistical interaction between OMT and UST based on moderate and substantial improvements in low back pain with each intervention. Any significant departure from T = 0 was indicative of interaction.
Secondary Outcomes
The distributions of secondary outcome measures also mandated use of nonparametric methods. The Mann-Whitney test was used to analyze RMDQ and SF-36 GH scores at weeks 4, 8, and 12. We dichotomized disability specific to low back pain (0 vs ≥1 lost work days in the past 4 weeks), satisfaction with back care (very satisfied vs any other response), and use of low back pain co-treatments (no use vs any use during the study).
Other Statistical Methods
Patient flow, treatment adherence, and safety were assessed by contingency table methods. Hypothesis testing was by intention to treat, with a 2-sided α = .05. Missing data were generally imputed using the last observation carried forward. For the multi-item RMDQ and SF-36 GH outcomes, however, we preferentially used responses acquired during a given encounter whenever possible to impute missing data for that encounter.29 Sensitivity analysis was conducted to assess the impact of missing data and robustness of our imputation methods. First, we conducted per-protocol analysis using only the 362 patients with complete data. Second, we conducted an alternate analysis in which any patient with 1 or more missed treatments was considered to be a nonresponder. We did not specify a priori subgroup analyses because of concerns about statistical power and confounding in such analyses.35 Statistical analyses were performed with SPSS 17.0.3 (SPSS Inc), using Epi Info 6.04d (Centers for Disease Control and Prevention) for low back pain treatment effects.
RESULTS
Patient Flow and Characteristics
Baseline patient characteristics were comparable across main effects groups (Table 1). The patient flow diagram displays similar allocation to physicians, follow-up, and treatment adherence among main effects groups (Supplemental Figure 1, available at http://annfammed.org/content/11/2/122/suppl/DC1). A total of 397 (87%) patients attended the final encounter at week 12.
Baseline Patient Characteristics by Main Effects Group
Low Back Pain Outcomes
The repeated measures analysis of variance failed to reject the hypothesis of no interaction between OMT and UST (P = .34). This analysis further showed significant reductions in pain scores on the visual analog scale over time with OMT compared with sham OMT (P = .002), but not with UST compared with sham UST (P = .99). Correspondingly, the change scores on the visual analog scale at 12 weeks for OMT patients (median = –18 mm, IQR = –31 to 0 mm) were significantly better than for sham OMT patients (median = –9 mm, IQR = –25 to 3 mm (P = .002). There was no statistical interaction between OMT and UST in assessing moderate (T = –0.04; 95% CI, –0.22 to 0.14; P = .63) or substantial (T = –0.05; 95% CI, –0.23 to 0.13; P = .61) improvements in low back pain.
Overall, 145 (63%) OMT patients vs 103 (46%) sham OMT patients reported moderate improvement at week 12 (RR = 1.38; 95% CI, 1.16-1.64; P <.001). Similarly, 114 (50%) OMT patients vs 79 (35%) sham OMT patients reported substantial improvement (RR = 1.41; 95% CI, 1.13-1.76; P = .002). By contrast, moderate improvement was observed in 128 (55%) UST patients vs 120 (54%) sham UST patients (RR = 1.02; 95% CI, 0.86-1.20; P = .85). Substantial improvement was observed in 103 (44%) UST patients vs 90 (41%) sham UST patients (RR = 1.09; 95% CI, 0.88-1.35; P = .43). The OMT treatment effects in chronic low back pain were also clinically relevant because they met or exceeded the Cochrane Back Review Group criterion for a medium effect size in all analyses for moderate to substantial improvements, including the sensitivity analyses (Table 2). Ultrasound therapy was not efficacious in any of these analyses.
Treatment Effects for Osteopathic Manual Treatment and Ultrasound Therapy in Chronic Low Back Pain
Secondary Outcomes
Neither OMT nor UST yielded significant improvements in RMDQ or SF-36 GH scores (Table 3). Sham UST patients were less likely than UST patients and OMT patients were less likely than sham OMT patients to report work disability because of low back pain at weeks 4 and 8, respectively. Neither of these groups sustained significant improvements at week 12, however. The OMT patients were more likely than sham OMT patients to report being very satisfied with their back care at all endpoints (P <.001). A total of 31 (13%) OMT patients vs 46 (20%) sham OMT patients reported using prescription drugs for low back pain during the study (use ratio = 0.66; 95% CI, 0.43-1.00; P = .048). The statistical significance of this finding persisted after simultaneously controlling for all other co-treatments.
Secondary Outcomes by Main Effects Group
Safety Profiles
Only 1 patient developed a contraindication to continued participation that was adjudicated to be possibly related to OMT. This contraindication involved recurrent back spasticity following OMT. There were 27 (6%) patients with adverse events (Supplemental Figure 1). Nine (2%) patients had a serious adverse event, none of which was definitely or probably related to a study intervention. There were no significant differences between the main effects groups in the frequency of adverse events or serious adverse events.
Adequacy of Patient Blinding
All 6 treatments were attended by 191 (83%) OMT patients vs 191 (85%) sham OMT patients (adherence ratio = 0.98; 95% CI, 0.90-1.06; P = .59), and by 192 (82%) UST patients vs 190 (86%) sham UST patients (adherence ratio = 0.96; 95% CI, 0.89-1.04; P = .36). At week 12, sham OMT patients reported a median change score on the visual analog scale of −9 mm (IQR = −25 to 3 mm) vs −13 mm (IQR = −27 to 1 mm) reported by sham UST patients.
DISCUSSION
This study shows that OMT is efficacious for short-term pain relief when used to complement other co-treatments for chronic low back pain. Responder analysis confirmed that OMT met or exceeded the Cochrane Back Review Group criterion for a medium effect size for both moderate and substantial improvements in low back pain. Thus, low back pain reductions with OMT were statistically significant and clinically relevant. The less frequent use of prescription drugs for low back pain reported by OMT patients further corroborates the clinical relevance of our low back pain outcomes. Notably, these drugs were prescribed by independent nonstudy physicians who were blinded or unaware that their patients were participating in our study. Moderate to substantial pain reductions, such as those observed with OMT, have been associated with decreased need for rescue medication.26 Another trial reported decreased medication use with OMT, but without corresponding efficacy in relieving low back pain.11 Our results may begin to explain why one-third of ambulatory, chronic problem visits for low back pain in the United States are provided by osteopathic physicians, and why they less frequently prescribe medications, such as nonsteroidal anti-inflammatory drugs, than allopathic physicians during such visits.1
There are concerns that chronic low back pain is often managed with costly and invasive treatments of questionable efficacy and safety.36 Our results support the efficacy and safety of OMT; however, they do not address its cost-effectiveness. Nevertheless, the OMT regimen of 6 treatments was within the guidelines developed in the United Kingdom by the National Institute for Health and Clinical Excellence, which recommend up to 9 spinal manipulation treatment sessions over 12 weeks.5 Our results may be generalizable to other manual therapies because several OMT techniques in our protocol have been accepted for low back pain treatment by professional associations representing chiropractors and physiotherapists.37 Biweekly maintenance treatments with spinal manipulation extend short-term low back pain reductions for 9 months.20 A systematic review suggests that low back pain reductions with OMT may extend up to 1 year.30 Thus, a larger trial is warranted to assess the efficacy and cost-effectiveness of OMT at long-term endpoints.
To our knowledge, this OMT trial is the largest ever conducted. Other strengths of our study include allocation concealment, similarity of baseline patient characteristics across treatment groups, blinding of outcome assessors, high levels of treatment adherence and outcomes reporting, and intention-to-treat analysis. Our analysis and interpretation of the primary outcomes was consistent with IMMPACT recommendations.24,38 We also collected data on concurrent low back pain co-treatments to pragmatically assess the effectiveness of OMT as it is provided in real-life settings (ie, as a complement, rather than an alternative, to self-care and usual care for low back pain).
There were limitations of our study. Comorbid conditions, work disability, and low back pain co-treatments were self-reported by patients, but were not verified through medical or employment records. Also, missing data had to be imputed for 13% of patients at the final encounter. Nevertheless, sensitivity analysis using 2 alternate approaches corroborated our low back pain outcomes.
Imperfect placebo treatments are common in low back pain trials.39 The factorial design limited our ability to assess the sham OMT and sham UST treatments for their independent placebo effects. According to a Cochrane Collaboration review,6 only 1 trial of spinal manipulation for chronic low back pain has ever evaluated patient blinding.40 The investigators in that trial reported a mean pain score reduction of 6 mm (16% from baseline) on a visual analog scale, with 6 sham manipulation treatments over 2 weeks, and concluded that blinding was adequate.40 Similarly, when extrapolated to a 100-mm visual analog scale with a standard deviation of 25 mm,31 a systematic review of 27 clinical trials reported a standardized mean effect corresponding to a pain reduction of 7 mm with placebo treatments.19 By comparison, our sham OMT patients achieved a median pain score reduction of 9 mm (20% from baseline) on a visual analog scale, with 6 treatments over 8 weeks. Nevertheless, some degree of unblinding remained possible despite these surrogate data on patient blinding.
In conclusion, the OMT patients achieved moderate to substantial improvements in low back pain, which met or exceeded the Cochrane Back Review Group criterion for a medium effect size. The OMT patients also reported less frequent concurrent use of prescription drugs. They did not, however, report corresponding improvements in back-specific functioning, general health, or work disability. The OMT regimen was safe, parsimonious, and well accepted by patients as demonstrated by high levels of treatment adherence and satisfaction with back care. By contrast, UST was not efficacious in relieving chronic low back pain.
Acknowledgments:
We thank Michael V. W. Bergamini, PhD, and Brian A. Gladue, PhD, for their critical comments on the manuscript. We also express our gratitude to the safety officers; members of the Data and Safety Monitoring Board; patients, study coordinators, and physicians; and staff of The Osteopathic Research Center.
Footnotes
-
Conflicts of interest: authors report none.
-
To read or post commentaries in response to this article, see it online at http://www.annfammed.org/content/11/2/122.
-
Trial registry: ClinicalTrials.gov NCT00315120.
-
Author contributions: Dr Licciardone has full access to all the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
-
Funding support: This study was funded by grants to J.C.L. from the National Institutes of Health–National Center for Complementary and Alternative Medicine (K24-AT002422) and the Osteopathic Heritage Foundation.
-
Role of sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
-
Previous presentations: First presented at Using Manual and Conventional Therapies to Enhance Musculoskeletal Health, April 27, 2012, Dallas-Fort Worth, Texas. Another presentation was given at the 9th International Conference on Advances in Osteopathic Research, September 15, 2012, London, England.
- Received for publication May 7, 2012.
- Revision received August 17, 2012.
- Accepted for publication August 31, 2012.
- © 2013 Annals of Family Medicine, Inc.