Abstract
PURPOSE Without supporting evidence, clinicians commonly recommend that warfarin be taken in the evening. We conducted a randomized controlled trial to evaluate the effect of administration time (morning vs evening) on the stability of warfarin’s anticoagulant effect.
METHODS A total of 236 primary care physicians serving 54 western Canadian communities mailed letters of invitation to all their warfarin-using patients. Eligible patients were community-dwelling warfarin users (any indication) with at least 3 months of evening warfarin use and no plans for discontinuation. Participants were randomized (by web-based allocation) to morning vs continued evening warfarin ingestion. We used the Rosendaal method to determine the proportion of time within therapeutic range (TTR) of the international normalized ratio (INR) blood test month 2 to 7 postrandomization vs the 6 months prerandomization. The primary outcome was the percent change in proportion of time outside target INR range (with an a priori minimum clinically important difference of ±20%). All analyses were intention to treat.
RESULTS Between March 8, 2015 and September 30, 2016, we randomized 109 participants to morning and 108 to evening warfarin use. TTR rose from 71.8% to 74.7% in the morning group, and from 72.6% to 75.6% in the evening group, for a change in TTR of 2.9% in the former vs 3.0% in the latter (difference, –0.1%; P = .97; 95% CI for the difference, –6.1% to 5.9%). The difference in percent change in proportion of time outside the therapeutic INR range (obtained via Hodges-Lehmann estimation of the difference in medians) was 4.4% (P = .62; 95% CI for the difference, –17.6% to 27.3%).
CONCLUSIONS Administration time has no statistically significant nor clinically important impact on the stability of warfarin’s anticoagulant effect. Patients should take warfarin whenever regular compliance would be easiest.
- chronotherapy
- warfarin
- Coumadin
- TTR
- anticoagulation
- atrial fibrillation
- thromboembolism
- mechanical valve
- primary care
- practice-based research
INTRODUCTION
Stroke and pulmonary embolism have devastating, often lifelong health consequences, and conditions that predispose to these events (atrial fibrillation, deep vein thrombosis, and mechanical heart valves) are common. Warfarin substantially reduces the risk of such thromboembolic events.1 The safety and effectiveness of warfarin depends greatly on the proportion of time in the therapeutic range (TTR) of the international normalized ratio (INR) blood test, however.2–5
Most physicians and pharmacists recommend warfarin be taken in the early evening.6–10 This strategy likely shortens the interval between learning of the need to make a dose adjustment (typically communicated to patients in the late afternoon following a morning blood test) and being able to make that dosing change. Hence, if evening warfarin use means quicker dose adjustments, it might conceivably lead to better TTR. Although the hypothesis is reasonable, there is no evidence to support this practice and other factors could meaningfully influence optimal administration time. For instance, dietary vitamin K (with which warfarin interacts) has an ultrashort 2.5-hour half-life and is found in foods (green leafy vegetables) having highly variable intake and rarely ingested in the morning.11,12 Conceivably, ingesting warfarin in the morning, when vitamin K levels are more consistent, might lead to greater INR stability. Although patients are commonly advised to take warfarin in the evening, it is unclear whether administration time matters, and if it does, which time is best.
In this pragmatic primary care study, The Effect of Warfarin Administration Time on Anticoagulation Stability (INRange), we randomized established warfarin users taking the medication in the evening either to switch to morning warfarin use or to continue evening use, and examined the TTR to detect differences in the stability of warfarin’s anticoagulant effect.
METHODS
Study Design and Setting
INRange was a prospective randomized, open, blinded-endpoint (PROBE)13 study carried out in the offices of 236 primary care clinicians serving 54 western Canadian communities. Most clinicians were community family physicians in full-time, fee-for-service practice who were practicing remotely from academic centers but affiliated with the Pragmatic Trials Collaborative14 (a multiprovincial practice-based research network). Patient participants were recruited with letters of invitation and study information packages that their primary care clinicians mailed to all warfarin users under their care. Interested patients called a contact number in the information package and were assessed by telephone for eligibility by a research assistant. A detailed study protocol and analytic plan have been published.15 No alterations to the registered or published protocol were made.
Participants
Trial inclusion criteria were (1) dinner or evening warfarin use; (2) community-dwelling status (assisted living residents were allowed to participate, but only if they had control of their own medication timing); (3) an expectation of long-term warfarin use; (4) availability of at least 3 months of baseline INR data (the last 6 months were used if available) with at least 4 evaluable INR results no more than 8 weeks apart from another INR reading.
Although no formal exclusion criteria were applied when patients were assessed for eligibility, clinicians were asked not to mail invitation letters to those they believed to have a limited life expectancy (less than 1 year) or to be incapable of providing informed consent.
All participants provided informed consent, either in writing or online.
Randomization and Masking
When the research team obtained adequate baseline INR data from the primary care clinician, consented eligible participants received a telephone call from a research assistant (with no clinical interactions) who obtained the participant’s allocation group over the web from the central research electronic data capture (REDCap)16 server’s randomization module (ensuring irreversible and concealed allocation). To minimize imbalance, the randomization sequence was stratified by percentage of readings within therapeutic range (<50%, 50% to 80%, >80%) and used variable blocks of 2 or 4. Study evaluators were blinded to allocation, but patients (who administered their own medications) were not blinded and free to share this information with their clinicians if either deemed it clinically relevant.
Procedures
This study was prospectively registered with ClinicalTrials.gov as NCT02376803 on February 25, 2015, before any patients were enrolled. Ethics approval was obtained from the clinical research ethics boards at the University of Alberta, and the University of British Columbia.
Data Collection
Baseline characteristics and information believed predictive of TTR were obtained directly from patients during a telephone interview immediately before randomization. These data included the self-reported average number of days per week that foods having a high vitamin K content (kale, spinach, chard, beet greens, broccoli, Brussels sprouts, romaine lettuce, or cabbage) were consumed, and how variable the participant felt this estimated level of vitamin K consumption was on a 4-point scale.
For follow-up, participants could choose between e-mail questionnaires generated by REDCap or telephone interviews, occurring 1 week, 1 month, and 7 months after either their timing change if they were in the intervention group (with the change made 5 days before the next scheduled INR test) or the date of randomization if they were continuing with evening use. During these interviews, participants self-reported compliance with allocation, as well as any illnesses and potentially warfarin-related adverse events (bleeding and thromboembolic events). Follow-up INR data for the 7 months postrandomization were obtained directly from primary care clinicians.
Warfarin Management
Patients’ clinicians continued to manage their warfarin therapy as per their usual routine, with no initial changes to dosing or any planned initial changes to the frequency of INR testing. In 152 of the 236 practices mailing out invitations, warfarin was managed solely by a family physician. In the remaining 84 practices warfarin was managed by clinic-affiliated nurses (74 practices) and pharmacists (10 practices), with consultation of the family physician as required.
Outcomes
The primary outcome was the percent change in the proportion of time spent outside the target INR range, with the minimum clinically important difference predefined to be ±20%. (The rationale for selecting this value and others is outlined below.) Secondary outcomes were the absolute change in TTR, the percent of patients with TTR above and below various cutoffs, the maximum and minimum out-of-range INR values, and the percent of INR values above and below the range.
As an exploratory outcome, we assessed for an interaction between warfarin timing and a patient’s self-assessment of the variability and number of days per week that foods with high vitamin K content were consumed in any amount.
All outcomes were as described in the registered and published protocols with no alterations.
Statistical Analyses
Minimum Clinically Important Difference
For patients with atrial fibrillation, an estimated 7% absolute improvement in TTR would prevent 1 major hemorrhage per 100 patient-years.4 Over the same period, an estimated 12% absolute improvement would prevent 1 thromboembolic event.4 These numbers are comparable to the 5% to 10% minimum clinically important difference for absolute change in TTR that was suggested by an informal sampling of clinicians,17 and are consistent with the statement from a randomized controlled trial that an observed (statistically significant) 6% absolute difference in TTR was “modest” and “less than the minimum clinically important difference” they had predefined (10%).18 We therefore chose 6% as our minimum clinically important difference for absolute change in TTR. A trial similar to ours (a Canadian primary care randomized controlled trial recruiting all warfarin users) reported a 70% baseline TTR.19 Expecting similar baseline characteristics (ie, that participants would be out of range 30% of the time at baseline), the corresponding minimum clinically important difference for percent change in proportion of time out of range was thus 6% out of 30% (ie, ±20%).
Sample Size
We determined that 170 participants (increased to 200 to account for potential dropouts and noncompliance) were needed to demonstrate a 20% difference in the percent change in proportion of time outside therapeutic INR range, assuming 90% power, an alpha error of .05, and a standard deviation twice the mean effect (ie, SD = 40%).
Calculating TTR
Target INR ranges vary by patient and by physician. Typically, the therapeutic range is 1 unit wide, usually either 2 to 3, or 2.5 to 3.5 depending on the indication for anticoagulation. It can, however, sometimes be narrower or wider (eg, 2 to 2.5, or 2 to 3.5). To standardize the width of each patient’s target INR range, we determined the midpoint of their individual target range and set their standardized target range to be 0.5 units above and below this midpoint (eg, a wider 2 to 3.5 target, with a midpoint of 2.75, would translate to a standardized target range of 2.25 to 3.25).
Primary and Secondary Analyses
All analyses were by intention to treat. If follow-up data were missing or insufficient, we assigned them the baseline value (ie, assumed no change). The primary outcome, percent change in proportion of time outside therapeutic INR range, could be analyzed only in patients for whom the proportion of time outside therapeutic range was nonzero at baseline. This situation occurs because the percent change in anything is not calculable when its baseline value is zero (since you cannot divide by zero). Hence, we assigned all patients who were never out of range at baseline the smallest observed nonzero baseline value (ie, treated them as being out of range 0.77% of the time). All statistical analyses were completed using PRISM 7.0d software (GraphPad Software) and all P values were 2-sided.
Comparison of Outcomes
For continuous outcomes, when comparisons of change from baseline were normally distributed, we used the difference in sample means to estimate effect size, and the Student t test to determine statistical significance. When outcomes were not normally distributed, statistical significance was determined by the Mann-Whitney U test and the Hodges-Lehmann estimate for difference in medians was used to determine effect size.
For dichotomous outcomes, the Fisher exact test was used to compare values, with confidence intervals calculated using the Koopman asymptotic score (for relative risk) and the Newcombe-Wilson score with continuity correction (for attributable risk).
Sensitivity Analysis
The percent change in the proportion of time that patients are out of range can have large swings when anticoagulation status is very well controlled. For example, if a patient has a 98% baseline TTR that falls to 90%, their proportion of time out of range (0.02 rising to 0.1) rises by 400%. The subset of patients with initially very well controlled anticoagulation could therefore disproportionately drive the analysis. To account for this possibility, we performed a sensitivity analysis for the primary outcome including only patients who were out of range more than 10% of the time (ie, having a TTR <90%).
Exploratory Analysis
A Kruskal-Wallis test (1-way ANOVA of ranks) was used to search for an interaction between warfarin timing and 2 patient-reported measures: average number of days per week that foods having high vitamin K content were consumed (<2 days, 2-5 days, >5 days), and variability in the consumption of those foods (a binary high or low variable constructed from 4 possible responses).
Data Availability
At the time of publication of this article, anonymized patient-level data, including all nonidentifying baseline characteristics and outcomes, will be made available to the public at http://www.PragmaticTrials.ca.
RESULTS
The study took place between March 8, 2015, and September 30, 2016, with 236 primary care physicians in 54 western Canadian communities mailing 2,107 recruitment letters. We assessed 351 patients for eligibility and excluded 134 before randomization (Figure 1). Of these, 93 were excluded because they already used warfarin in the morning, 10 were reluctant to change medication timing if randomized to do so, and 10 had baseline INR data that were either insufficient or unavailable. Other reasons for exclusion are shown in the trial flow diagram.
CONSORT flow diagram.
CONSORT = Consolidated Standards of Reporting Trials; INR = international normalized ratio.
Of the 217 participants (10.3% of those to whom letters were mailed), 109 were randomized to switch to morning warfarin and 108 we randomized to continue evening use as a control. The total exceeded the sample size of 200 needed to demonstrate our 20% minimum clinically important difference. Patients’ baseline characteristics are shown in Table 1. For the intervention group, the rate of nonadherence to allocation (2 patients) and missing data (2 patients) was 3.7% combined, as compared with 1.9% in the control group.
Patients’ Baseline Characteristics by Treatment Group
The percent change in the proportion of time patients were outside therapeutic INR range was not normally distributed (D’Agostino-Pearson normality tests <.0001). The Hodges-Lehmann estimator for the difference in medians (morning vs evening) was 4.4% (P = .62; 95% CI for the difference, –17.6% to 27.3%) with actual medians of –22.9% vs –11.9%. This 4.4% difference was substantially less than our predefined ±20% minimum clinically important difference.
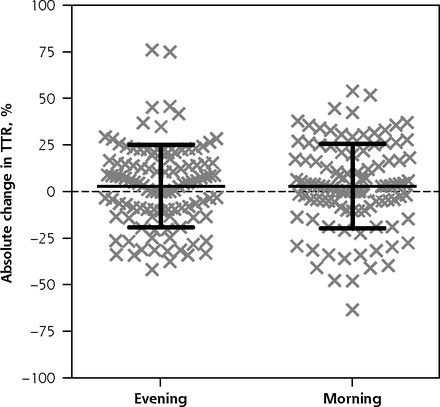
The absolute change in TTR was normally distributed (Figure 2), with a value of 2.9% for the morning group vs 3.0% for the evening group (P = .97; 95% CI for the difference, –6.1% to 5.9%). Maximum and minimum INR, and percent of readings above and below therapeutic range (Table 2) were not normally distributed.
Scatterplot of absolute change in TTR.
TTR = time within therapeutic range (of the international normalized ratio).
Note: Each of the 217 data points represents the absolute change in TTR for 1 study participant. The overlaid black crossbars indicate mean and SD.
Anticoagulation Outcomes at 7 Months
Major warfarin-related adverse cardiovascular events in the morning group included 1 gastrointestinal bleeding event, 1 thromboembolic stroke, and 1 deep vein thrombosis. These numbers compared with 1 thromboembolic stroke and 1 deep vein thrombosis in the evening group (who also had the only other acute arterial occlusion, a myocardial infarction).
Neither the self-reported number of days per week that foods having high vitamin K content were consumed (P = .79) nor the patient’s global estimate of the variability of those foods within the diet (P = .72) influenced the effect of the intervention on the primary outcome.
In our sensitivity analysis comparing the primary outcome in the 84 morning group and 85 evening group participants with baseline TTR of less than 90%, the Hodges-Lehmann difference in medians, which now trended in the opposite direction, was –5.3% (P = .49; 95% CI for the difference, –25.8% to 11.3%).
DISCUSSION
Our results show that warfarin administration time, morning vs evening, has no clinically important effect on the proportion of time that warfarin users spend outside the therapeutic range of the INR blood test. We found this to be true regardless of the self-reported frequency and variability with which foods containing high amounts of vitamin K were consumed.
Although it is a strength that our study participants (and their clinicians) were recruited from a geographically broad primary care population, baseline TTR for the group as a whole was slightly higher (mean, 72.2%) than that achieved in a nationally representative sample of Canadian primary care practices (mean, 67.8%).20 Conceivably, those who volunteered might be more compliant or healthier than average, with less opportunity for an intervention to demonstrate benefit. Our primary outcome is also a limitation in that patients with excellent baseline control can disproportionately drive it. Absolute change in TTR was, however, nearly identical in both groups, and we found literature-derived minimum clinically important differences that are substantially larger than the point estimates for both our primary outcome (observed change, 4.4%; minimum clinically important difference ±20%), and the absolute change in TTR (observed change, –0.1%; minimum clinically important difference, ±6%). TTR is also limiting in that it is a surrogate outcome. Our study was not powered to examine differences in clinical events.
To our knowledge, although patient-facing information commonly suggests warfarin should be taken in the evening,6–10 there is no evidence to support this practice. Of 1,642 articles returned in our literature review (searching PubMed with the term warfarin [MESH] and filtering for clinical trials), none examined the influence of warfarin administration time. We believe our study is the first to address this question.
The lack of a dietary interaction, and the apparent absence of any effect of warfarin administration time on INR stability might be explained, at least in part, by the 36- to 42-hour half-life of racemic warfa-rin.11 It is also possible that the benefit on TTR is real, but only observable with ultrashort testing intervals that weekly (or longer) INR testing would miss. If we assume true benefit equivalent to the 12-hour lead time for dosing changes that evening (vs morning) administration might bring about, however, it would have very little effect on TTR. In our experience, patients seldom exceed 2 dose changes per month. If each (evening) dose change provided 12 extra hours in range, that would provide at best an extra 24 hours in range per month, a clinically unimportant improvement in TTR of 3.3% or less.
Although direct oral anticoagulants (DOACs) have begun replacing warfarin in recent years, warfarin is superior to DOACs, on both thromboembolic and bleeding outcomes, in patients with mechanical heart valves.21,22 Hence, it is likely to remain the anticoagulant of choice in that population. Given warfarin is also much less expensive than DOACs, and may provide similar outcomes in patients with TTR greater than 65%,23,24 it will also remain a viable option when cost is a concern to patients, especially in countries where mean TTR exceeds 65% (eg, Sweden, United Kingdom, Canada).20,25,26
We have found the percent change in time outside therapeutic INR range resulting from morning vs evening warfarin administration to be neither statistically significant nor clinically important should the observed difference be real. Despite common and widespread advice to the contrary, warfarin administration time does not appear to be clinically important. Given that patients and their community caregivers might find a particular time of day more convenient or easier to remember, warfarin administration time should be tailored to patient preference.
Acknowledgments
We would like to thank the many medical office assistants, and other primary care team members, who devoted time to preparing and mailing recruitment letters—in particular, the INR nurses of the Oliver Primary Care Network. We would also like to thank both Dorna Sadeghi, who assisted with data analysis, and the volunteer family physicians of the Pragmatic Trials Collaborative, who enabled this work to take place in their community practices.
Footnotes
Conflicts of interest: authors report none.
To read or post commentaries in response to this article, see it online at http://www.AnnFamMed.org/content/18/1/42.
Funding support: Funding for this work came solely from nonprofit sources including the Vancouver Coastal Health Research Institute, the research arm of a regional health authority (https://www.vchri.ca/about-us); the Richmond Hospital Foundation, a community hospital foundation (http://www.richmondhospitalfoundation.com/home); and EnACt, a government-funded organization fostering primary care research (https://primarycareresearch.ca/).
Disclaimer: The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the paper, or the decision to submit for publication.
Previous presentation: This work was previously presented at the North American Primary Care Research Group (NAPCRG) 2018 Annual Meeting; November 12, 2018; Chicago, Illinois.
Trial registration: NCT02376803
- Received for publication January 19, 2019.
- Revision received May 5, 2019.
- Accepted for publication June 12, 2019.
- © 2020 Annals of Family Medicine, Inc.