Abstract
BACKGROUND We wanted to determine the type of outpatient medical care reported by young adult survivors of childhood cancer and to examine factors associated with limited medical care.
METHODS We analyzed data from 9,434 adult childhood cancer survivors enrolled in a retrospective cohort study who completed a baseline questionnaire. They had a mean age of 26.8 years (range 18 to 48 years), 47% were female, 12% were minorities, and 16% were uninsured. Four self-reported outcome measures were used to determine outpatient medical care in a 2-year period: general contact with the health care system, general physical examination, cancer-related medical visit, and medical visit at a cancer center.
RESULTS Eighty-seven percent reported general medical contact, 71.4% a general physical examination, 41.9% a cancer-related visit, and 19.2%, a visit at a cancer center. Factors associated with not reporting a general physical examination, a cancer-related visit, or a cancer center visit included no health insurance (odds ratio [OR] = 2.34; 95% confidence interval [CI], 1.97–2.77), male sex (OR = 1.65; 95% CI, 1.44–1.88), lack of concern for future health (OR = 1.57; 95% CI, 1.36–1.82), and age 30 years or older in comparison with those 18 to 29 years (OR = 1.56; 95% CI, 1.35–1.81). The likelihood of reporting a cancer-related visit or a general physical examination decreased significantly as the survivor aged or the time from cancer diagnosis increased. This trend was also significant for those treated with therapies associated with substantial risk for cardiovascular disease or breast cancer.
CONCLUSIONS Primary care physicians provide health care for most of this growing high-risk population. To optimize risk-based care, it is critical that cancer centers and primary care physicians develop methods to communicate effectively and longitudinally.
INTRODUCTION
With improvements in treatment during the recent decades, survival rates for childhood cancer have increased to more than 70%, resulting in an increasing population of long-term survivors.1 Currently, 1 in 900 young adults is a childhood cancer survivor.2 With this growth in survivorship, there has been a developing recognition of the potential long-term health problems related to cancer therapy. All organ systems can be affected by radiation, chemotherapy, or surgery, leading to a wide array of potential late effects.
Long-term survivors of childhood cancer face considerable risk for late mortality, morbidity, and adverse health status secondary to their previous cancer therapy. In a retrospective analysis of 20,227 childhood cancer patients who had survived 5 years, Mertens et al3 reported an absolute excess risk for mortality from second cancers (not including late recurrences), cardiac causes, and pulmonary causes of 1.26, 0.27, and 0.015 deaths, respectively, per 1,000 person-years. Morbidity secondary to late effects of chemotherapy or radiation is frequent and often serious. In 5 studies reporting on long-term survivors with a median age ranging from 15 to 23 years, 58% to 69% had at least one late effect of therapy, with 25% to 30% experiencing a moderate to severe late complication.4– 8 Hudson et al9 found that 44% of adult survivors of childhood cancer reported at least one domain of their health status to be moderately to extremely adversely affected.
Survivors can benefit from early diagnosis and intervention or preventive care targeted at reducing risk for late effects, such as second malignant neoplasms of the breast,10, 11 thyroid,12, 13 and skin14, 15 after radiation therapy; altered bone metabolism and osteoporosis16, 17; obesity-related health problems (dyslipidemia, hypertension, diabetes mellitus, cardiovascular disease)18– 20; liver failure secondary to chronic hepatitis C after blood transfusion21; and endocrine dysfunction after chest-mantle radiation.22, 23 Longitudinal care addressing other late effects, such as infertility, musculoskeletal problems, cognitive dysfunction, and psychosocial issues, may also improve survivors’ health outcomes and quality of life.
Most late effects increase in incidence with age, often becoming clinically apparent decades after therapy. Because this window of time offers the potential to modify severity of health outcomes by prevention or early intervention, there is consensus that survivors of childhood cancer should have longitudinal risk-based health care.4– 8, 24– 26 In a recent report, Ensuring Quality Cancer Care,27 the National Cancer Policy Board, established through the Institute of Medicine, recommended lifelong follow-up of all cancer survivors as a key component to improving the quality of cancer care. Expert opinion, based on limited evidence, recommends that risk-based health care include a systematic plan for screening, surveillance, and prevention, incorporating a survivor’s risks based on the previous cancer or cancer therapy, genetic predispositions, lifestyle behaviors, and comorbid health conditions.24– 26, 28
To date, the health care utilization patterns of young adult survivors have not been reported. It is unknown what percentage seek or receive medical care related to their previous cancer, and whether this care is risk based. The purpose of this exploratory study was to use a large cohort of young adult survivors to (1) determine the type of outpatient medical visits that survivors report in a 2-year period, and (2) examine factors associated with limited follow-up or medical visits.
METHODS
Subjects
The Childhood Cancer Survivor Study (CCSS) is a multi-institutional study of patients who survived for 5 or more years after diagnosis of a cancer. Eligibility criteria for the cohort included (1) confirmed diagnosis of 1 of the 8 primary cancer groups (leukemia, central nervous system malignancies, Hodgkin’s disease, non-Hodgkin’s lymphoma, kidney tumor, neuroblastoma, soft tissue sarcoma, or bone tumor); (2) diagnosis and initial treatment at 1 of the 25 collaborating institutions; (3) diagnosis between January 1, 1970, and December 31, 1986; (4) age less than 21 years at diagnosis; and (5) survived at least 5 years from diagnosis. The total eligible sample fulfilling these criteria was 20,276. Relying upon the last known address provided by the treating institution, a total of 7,913 (39%) required tracing to locate the eligible survivor or his or her parents. Of those survivors who required tracing, 4,917 (62%) were located; 2,996 (38%) were not despite intensive tracing efforts and were subsequently classified as lost to follow-up. The result was a total sample of 17,280 eligible survivors who were contacted regarding study participation.
The CCSS protocol and contact documents were reviewed and approved by the Human Subjects Committee at each participating institution. Baseline data were collected by mail or telephone for members of the study cohort using a 24-page questionnaire that was designed to capture a wide range of information, including sociodemographic characteristics, health habits, chronic medical conditions, and access and utilization of medical care. Information on the characteristics of the original cancer diagnosis was obtained from the treating institution. For all CCSS respondents who returned a signed medical release, information concerning primary cancer therapy and initial treatment was collected. Copies of the baseline questionnaire and the treatment abstraction form used in data collection are available for review and can be downloaded at http://www.cancer.umn.edu/ccss. Further details regarding the methodology and cohort characteristics were published previously.29
Outcome Measures
Four dichotomous outcome measures were used to describe different types of outpatient medical care reported by survivors in a 2-year period: (1) general or nonspecific contact with a health care provider, (2) general physical examination, (3) cancer-related medical visit, and (4) medical visit at a cancer center. These outcomes were not mutually exclusive. To ascertain general or nonspecific medical contact, respondents were asked whether they had contact with a physician, nurse, or other health care provider. Such contact could include a visit to a physician’s office or a telephone call. To ascertain cancer-related medical visits and cancer center visits, respondents were asked how many of the visits to a physician’s office were related to their previous cancer and whether any of the visits were at an oncology (cancer) center. The content or additional details about the medical visits were not ascertained, and it is possible that they incorporated screening related to the previous cancer treatment.
Independent Variables
Sociodemographic variables included age at time of interview, sex, race and ethnicity, education, health insurance, and household income. Because the number of black, non-Hispanic and Hispanic survivors was small, they were combined with other race and ethnic minorities and analyzed as a single group. Cancer-related variables included cancer type, age at diagnosis, time from cancer diagnosis to baseline questionnaire, subjective health status, pain or anxiety as a result of the cancer or its treatment, and concern for future health. To assess follow-up patterns for survivors who might be at higher risk for late effects, a high-risk treatment variable was created that consisted of those cases of patients who received one or more of the following treatments: radiation to the mantle or chest, anthracycline with a cumulative dose ≥ 300 mg/m2, bleomycin, ifosfamide, or etoposide.
Analysis
Univariate analyses were performed to assess the associations of demographic and cancer-related variables with the medical care outcome measures. The Cochran-Armitage trend test was used for assessing trends in binomial proportions with aging or increasing time from cancer diagnosis. To determine the strength of association between the outcome variables and the demographic and cancer-related factors hypothesized to be significant a priori, regression analysis was used to estimate odds ratios (OR) with 95% confidence intervals (CI) for absence of each outcome. Specifically, generalized linear mixed models (logistic regression with institution-specific Gaussian random intercepts) were used to account for possible clustering based on institution. Data were analyzed with the SAS version 8 (SAS institute, Cary, NC) with 2-tailed statistical tests.
RESULTS
Of the 17,280 study subjects contacted, 14,054 (81%) responded by completing the baseline questionnaire. At the time of this analysis, 9,434 were alive and aged 18 years or older at interview. The demographics and cancer treatment of the 9,434 adult survivors are provided in Tables 1⇓ and 2⇓. The mean age at time of baseline questionnaire was 26.8 years (range 18 to 48 years), and the mean interval from diagnosis to completion of questionnaire was 17.4 years (range 6.4 to 29.4 years).
Percentage of Adult Survivors of Childhood Cancer Who Reported the Following Types of Outpatient Medical Care in a 2-Year Period: Sociodemographic Factors
Percentage of Adult Survivors of Childhood Cancer Who Reported the Following Types of Outpatient Medical Care in a 2-Year Period: Cancer-Related Factors
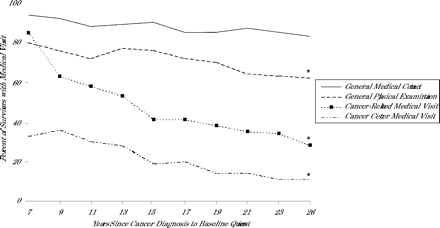
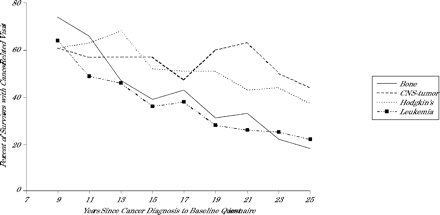
Tables 1 ⇑and 2⇑ show the percentage of respondents who reported the 4 types of medical visits by each of the demographic and cancer-related variables. The older the survivor, the less likely he or she was to report a general physical examination (P <.001), a cancer-related visit (P <.001), or a visit to a cancer center (P <.001). Similarly, as the interval from cancer diagnosis increased, the survivors were less likely to report these types of visits (P <.001 for each outcome; Figure 1⇓). For survivors who reported a general physical examination, the likelihood of also reporting a cancer-related visit decreased with age (P <.001) or with an increasing interval from cancer diagnosis (P <.001). The longer the interval from cancer diagnosis, the less likely survivors from 4 high-risk cancer groups (bone tumor, central nervous system tumor, Hodgkin’s disease, leukemia) were to report a cancer-related visit (P <.001 for each group; Figure 2⇓). Table 3⇓ provides the final multivariate models of factors associated with limited contact or care. Findings in addition to those reported in Table 3⇓ are described below.
Multivariate Risk Factors for Absence of the Following Types of Outpatient Medical Care in a 2-Year Period in Adult Survivors of Childhood Cancer
Percentage of adult survivors of childhood cancer with medical visits in a 2-year period by interval from cancer diagnosis to baseline questionnaire.
* Trend significant with P <.001 for general physical education, cancer-related medical visit, and cancer center medical visit by Cochran-Armitage trend test.
Percentage of adult survivors of 4 higher risk cancer groups with a cancer-related medical visit in a 2-year period by interval from cancer diagnosis to baseline questionnaire.
Note: Trend significant with P <.001 for bone tumor, central nervous system (CNS) tumor, Hodgkin’s disease, and leukemia survivors by Cochran-Armitage trend test.
Survivors who had received high-risk treatments were significantly more likely to have each type visit than other survivors (Table 4⇓). As above, the likelihood of a general physical or a cancer-related visit decreased with age (P <.001 for both types of visits) or as the interval from cancer diagnosis increased (P <.001 for both types of visits).
Percentage of Adult Survivors of Childhood Cancer Treated With Therapies Associated With Increased Risk for Late Effects Reporting an Absence of the Following Types of Outpatient Medical Care in a 2-Year Period, Compared With All Survivors
Twenty-percent reported not having a general physical examination, a cancer-related visit, or a visit at a cancer center. A multivariate model estimated the following odds ratios for lack of at least 1 of these 3 types of medical visits: survivors 30 years or older in comparison with those less than 29 years of age (OR = 1.56; 95% CI, 1.35–1.81), male sex (OR = 1.65; 95% CI, 1.44–1.88), survivors without health insurance (OR = 2.34; 95% CI, 1.97–2.77), lack of concern for future health (OR = 1.57; 95% CI, 1.36–1.82), and high-risk treatment (OR = 0.64; 95% CI, 0.55–0.73).
DISCUSSION
This study is the first to report general and cancer-related health care visits in young adult survivors of childhood cancer in the United States and Canada. Four primary findings are discussed below: (1) most survivors reported some contact with the medical system, (2) the likelihood of a general physical examination or a cancer-related medical visit decreased substantially as the survivor aged or the time interval from diagnosis increased, (3) less than 20% of survivors were seen in a cancer center, and (4) risk-based health care of adult survivors of childhood cancer is likely uncommon.
Almost 90% of adult survivors reported some contact with a health care clinician in the 2-year period, suggesting that access to general or nonspecific health care might not be an important issue for most young adult survivors in this cohort. Factors typically associated with lack of health care in the general population,30– 33 such as lack of medical insurance, ethnic minority status, and male sex, were associated with limited nonspecific medical contact.
About 75%, 50%, and 25% of survivors in their younger adult years, 18 to 24 years, reported a general physical examination, a cancer-related visit, or a visit to a cancer center, respectively. The proportion of survivors reporting these types of visits decreased significantly with age and with increasing time from cancer diagnosis. This decrease in health care utilization occurred at a stage in life when the incidence of many late effects of cancer therapy, including most second cancers, cardiovascular disease, osteoporosis, and endocrinopathies, are increasing. Encouragingly, those who needed follow-up the most, survivors treated with high-risk therapies, were more likely than other survivors to report a general physical examination, a cancer-related visit, or a visit to a cancer center. Even in this high-risk group, however, the likelihood of visits decreased significantly with age or increasing interval from the cancer diagnosis. Of note, ethnic or racial minority status was not associated with absence of these types of visits. In contrast, male and uninsured survivors were less likely to report such visits.
Although the content of these types of medical visits was not assessed in this study, 2 examples of medical care may be illustrative. Treatment with an anthracycline, used in the therapy for several childhood cancers, can lead to a late-onset cardiomyopathy, often occurring 10 to 20 years after the cancer therapy.34, 35 The optimum and cost-effective methods to screen for left ventricular wall motion abnormalities are still being studied, but there is consensus that survivors who were treated with 7ge;300 mg/m2 of an anthracycline should be observed closely longitudinally.34, 35 In this analysis, only 51.7% of survivors who had been treated with ≥300 mg/m2 of an anthracycline reported a cancer-related medical visit.
Care reported by Hodgkin’s disease survivors, the cancer group that faces perhaps the most serious risks of childhood cancer survivors, serves as a second example. Depending on therapy, Hodgkin’s disease survivors face a markedly increased risk for second cancers,10– 13, 36, 37 endocrine dysfunction,12 coronary artery disease,38, 39 cardiac valvular disease,40 ventricular dysfunction,35 infertility,41 and premature menopause.42 Though Hodgkin’s disease survivors in this study were more likely to report cancer-related care than other survivors, nearly 50% reported no such care. As with the general survivor population, those in these 2 high-risk groups were less likely to report either a general physical examination or a cancer-related visit as they aged or as the time from diagnosis increased.
These findings have important implications for cancer centers and primary care physicians. Whereas the incidence of many modifiable late effects increases with age, the likelihood of survivors having general physical examinations or cancer-related care appears to decrease, which implies that provision of risk-based care also decreases with time. There are 4 key obstacles contributing to this problem: many cancer centers do not provide survivors with adequate information about late effects, most survivors are unaware of their risks, primary care physicians are unfamiliar with the population, and there is little formal communication between cancer centers and primary care physicians. In the 1980s, with the burgeoning information regarding late effects of therapy, long-term follow-up programs were established in many cancer centers with the intent of providing survivors with screening, surveillance, and education about late effects. Transition of medical care for adolescent and young adult survivors from long-term follow-up programs to primary care physicians has been recommended.24– 26 By 1997, however, only 53% of childhood cancer centers in North America had developed a long-term follow-up program.43 Thus, most young adult survivors have not been seen in such a program.44 Even though most survivors have a fair knowledge of their general diagnosis and whether they received chemotherapy or radiation therapy, few have a summary of their treatment or are aware of their risks for late effects of therapy.44
Although primary care physicians provide general health care for most survivors, there has not been a national effort to foster linkages between childhood cancer centers and primary care physicians to enhance risk-based care. There is a paucity of information about this population in primary care-based journals,45 and there is no mention of this population in primary care textbooks, resident curricula, or continuing medical education monographs.28 Finally, compounding this lack of dissemination of information, survivors represent a small percentage of a typical primary care physician’s practice.45 In addition, providing appropriate risk-based care to survivors, who are a heterogeneous group, is complicated by the variety of cancers diagnosed at different ages and treatment eras and by recommendations for screening and surveillance that are constantly evolving. As a result, there is a critical need for cancer centers, primary care physicians, and survivors to communicate and share information.
Readers are directed to http://www.cancer.umn.edu/ltfu for general information regarding risks of this population (see project newsletters). It is important that recommendations for risk-based care, based on current evidence, be disseminated in primary care journals and textbooks. As an example, Oeffinger et al46 provided recommendations for primary care physicians caring for leukemia survivors. To optimize risk-based care of this vulnerable population, interventions to educate survivors, enhance their transition to primary care physicians, and foster ongoing communication between survivors, cancer centers, and primary care physicians need to be developed and tested.
There are several limitations of this study that are important to consider when interpreting the findings. First, health care utilization was based upon self-report by respondents and was not externally verified. Second, the determination of cancer-related medical visits was based upon the survivor’s perception of the reasons for the medical visit. There are times when a health care professional might have seen a survivor and screened for late effects of therapy without the survivor understanding the rationale for testing. Similarly, the content of the visit as it pertained to appropriate risk assessment and screening based upon previous therapy and modifying factors, such as unhealthy behaviors or genetic predisposition for different diseases, was not determined from whether a survivor had a general physical examination or a cancer-related visit. Third, though nationally 25% of new childhood cancers are in patients of ethnic minorities,47 only 12.2% of this survivor cohort belonged to an ethnic minority. Because the collaborating institutions did not regularly collect race and ethnicity information on all patients treated between 1970 and 1986, it cannot be determined whether this low percentage of ethnic minorities represents a selection bias, a limitation in generalizability, or a lower proportion of minority survivors treated at these institutions.
Despite these limitations, our findings likely overestimate the percentage of long-term survivors who are receiving risk-based health care. Completion of the lengthy questionnaire required a degree of sophistication and interest on the part of the responding survivors, and it is probable that the nonrespondents are even less likely to have adequate risk-based health care.
In conclusion, though most adult survivors of childhood cancer in this large cohort study reported some type of contact with the medical system, the likelihood of a cancer-related visit or a physical examination decreased at an age when the incidence of modifiable late effects are increasing. Primary care physicians provide health care for most of this growing high-risk population. To optimize risk-based care, it is critical that cancer centers and primary care physicians develop methods to communicate effectively and longitudinally.
Childhood Cancer Survivor Study Institutions and Investigators
Institutional Principal Investigators: Arthur Ablin, MD, University of California-San Francisco, Calif; Roger Berkow, MD, University of Alabama, Birmingham, Ala; George R. Buchanan, MD, UT-Southwestern Medical Center at Dallas, Tex; Lisa Diller, MD, Dana-Farber Cancer Institute, Boston, Mass; Zoann Dreyer, MD, Texas Children’s Center, Houston, Tex; Debra Friedman, MD, MPH, Children’s Hospital and Medical Center, Seattle, Wa; Daniel M. Green, MD, Roswell Park Cancer Institute, Buffalo, NY; Mark Greenberg, MB, ChB, Hospital for Sick Children, Toronto, Ontario; Robert Hayashi, MD, St. Louis Children’s Hospital, Mo; Melissa Hudson, MD, St. Jude Children’s Research Hospital, Memphis, Tenn; Raymond Hutchinson, MD, University of Michigan, Ann Arbor, Mich; Michael P. Link, MD, Stanford University School of Medicine, Stanford, Calif; Brian Greffe, Children’s Hospital, Denver, Colo; Amanda Termuhlen, MD, Columbus Children’s Hospital, Ohio; Gregory Reaman, MD, Children’s National Medical Center, Washington, DC; A. Kim Ritchey, MD, Children’s Hospital of Pittsburgh, Pa; Leslie L. Robison, PhD, University of Minnesota, Minneapolis, Minn; Kathy Ruccione, RN, MPH, Children’s Hospital Los Angeles, Calif; Charles Sklar, MD, Memorial Sloan-Kettering Cancer Center, New York, NY; W. Anthony Smithson, MD, Mayo Clinic, Rochester, Minn; Louise Strong, MD, U.T.MD Anderson Cancer Center, Houston, Tex; Terry A. Vik, MD, Riley Hospital for Children, Indianapolis, Ind; Yutaka Yasui, PhD, Fred Hutchinson Cancer Center, Seattle, Wa; Lonnie Zeltzer, MD, University of California-Los Angeles, Calif.
Former Institutional Principal Investigators: Holcombe Grier, MD, Dana-Farber Cancer Institute, Boston, Mass; Thomas Pendergrass, MD, Children’s Hospital and Medical Center, Seattle, Wa; Teresa Vietti, MD, St. Louis Children’s Hospital, Mo; Lorrie Odom, MD, Children’s Hospital, Denver, Colo; Frederick Ruymann, MD, Columbus Children’s Hospital, Ohio; Julie Blatt, MD, Children’s Hospital of Pittsburgh, Pa; Gerald Gilchrist, MD, Mayo Clinic, Rochester, Minn; Robert Weetman, MD, Riley Hospital for Children, Indianapolis, Ind; John Potter, MD, PhD, Fred Hutchinson Cancer Center, Seattle, Wa.
Member CCSS Steering Committee: John Boice, ScD, International Epidemiology Institute, Rockville, MD; Norman Breslow, PhD, University of Washington, Seattle, Wa; Kevin Oeffinger, MD, UT-Southwestern Medical Center at Dallas, Tex; Frederick Li, MD, Dana-Farber Cancer Institute, Boston, Mass; Daniel M. Green, MD, Roswell Park Cancer Institute, Buffalo, NY; Melissa Hudson, MD, St. Jude Children’s Research Hospital, Memphis, Tenn; Anna Meadows, MD, Bobbie Bayton, Children’s Hospital of Philadelphia, Pa; John Mulvihill, MD, Children’s Hospital, Oklahoma City, Okla; Stephen Qualman, MD, Columbus Children’s Hospital, Ohio; Roger Packer, MD, Children’s National Medical Center, Washington, DC; Leslie L. Robison, PhD, Ann Mertens, PhD, Joseph Neglia, MD, MPH, Mark Nesbit, MD, Stella Davies, MD, PhD, University of Minnesota, Minneapolis, Minn; Charles Sklar, MD, Memorial Sloan-Kettering Cancer Center, New York, NY; Malcolm Smith, MD, Martha Linet, MD, National Cancer Institute, Bethesda, Md; Louise Strong, MD, Marilyn Stovall, PhD, U.T.MD Anderson Cancer Center, Houston, Tex; John Potter, MD, PhD, Fred Hutchinson Cancer Center, Seattle, Wa; Lonnie Zeltzer, MD, University of California-Los Angeles, Calif.
Acknowledgments
The authors would like to thank Pauline Mitby, MPH, for her assistance with the CCSS data base and Sandra Mulkey, RD, for her assistance with manuscript preparation.
Footnotes
Conflicts of interest: none reported
Funding support: This work was supported by Grant 5U01-CA-55727-05 from the Department of Health and Human Services and funding to the University of Minnesota from the Children’s Cancer Research Fund. Dr. Oeffinger received partial support for this work through the Robert Wood Johnson Foundation Generalist Physician Faculty Scholars Program and the American Academy of Family Physicians Advanced Research Training Program.
- Received for publication December 13, 2002.
- Revision received January 31, 2003.
- Accepted for publication February 18, 2003.
- © 2004 Annals of Family Medicine, Inc.