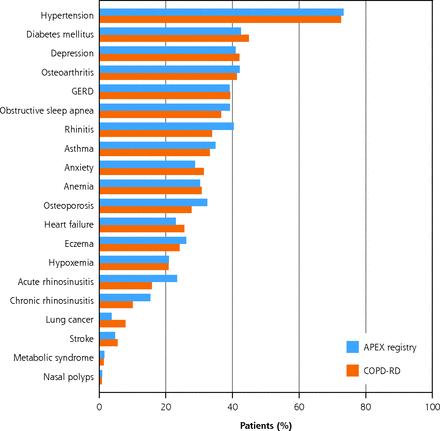Abstract
PURPOSE To describe demographic and clinical characteristics of chronic obstructive pulmonary disease patients managed in US primary care.
METHODS This was an observational registry study using data from the Chronic Obstructive Pulmonary Disease (COPD) Optimum Patient Care DARTNet Research Database from which the Advancing the Patient Experience COPD registry is derived. Registry patients were aged ≥35 years at diagnosis. Electronic health record data were collected from both registries, supplemented with patient-reported information/outcomes from the Advancing the Patient Experience registry from 5 primary care groups in Texas, Ohio, Colorado, New York, and North Carolina (June 2019 through November 2020).
RESULTS Of 17,192 patients included, 1,354 were also in the Advancing the Patient Experience registry. Patients were predominantly female (56%; 9,689/17,192), White (64%; 9,732/15,225), current/ex-smokers (80%; 13,784/17,192), and overweight/obese (69%; 11,628/16,849). The most commonly prescribed maintenance treatments were inhaled corticosteroid with a long-acting β2-agonist (30%) and inhaled corticosteroid with a long-acting muscarinic antagonist (27%). Although 3% (565/17,192) of patitents were untreated, 9% (1,587/17,192) were on short-acting bronchodilator monotherapy, and 4% (756/17,192) were on inhaled corticosteroid monotherapy. Despite treatment, 38% (6,579/17,192) of patients experienced 1 or more exacerbations in the last 12 months. These findings were mirrored in the Advancing Patient Experience registry with many patients reporting high or very high impact of disease on their health (43%; 580/1,322), a breathlessness score 2 or more (45%; 588/1,315), and 1 or more exacerbation in the last 12 months (50%; 646/1,294).
CONCLUSIONS Our findings highlight the high exacerbation, symptom, and treatment burdens experienced by COPD patients managed in US primary care, and the need for more real-life effectiveness trials to support decision making at the primary care level.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) represents an important public health challenge that is preventable and treatable.1 For the 16 million Americans who live with it, COPD carries a high morbidity, mortality, and socioeconomic burden.2-8 Adults with COPD are more likely to report inability to work, limitations to activity, and to suffer from more comorbid chronic disease, compared with adults without COPD.2,3 COPD is the third leading cause of death in the United States; more than 140,000 Americans die from it each year (1 every 4 minutes).5 Indeed, the COPD death rate has doubled since 1969, while death rates for other chronic diseases have declined.6 The 20-year (2019-2038) direct medical costs and indirect absenteeism costs associated with COPD in the United States are estimated at $800.9 billion and $101.3 billion, respectively.7 Globally, the COPD burden is projected to increase because of continued exposure to COPD risk factors, rising obesity, and increase in the proportion of women within an aging population.9
In the United States, approximately 80% of patients diagnosed with COPD are managed by their family physician or general internist.10 Despite the efforts of several campaigns to increase awareness of this disease and best clinical practice in primary care,11-13 many US family physicians and other primary care clinicians are unfamiliar with COPD management guidelines.14 Additionally, management guidelines may not reflect routine clinical practice, as the majority of their evidence is derived from randomized controlled trials (RCTs) rather than real-life effectiveness studies. The World Health Organization has called for more epidemiological studies to more accurately estimate the burden of chronic respiratory disease and encourages countries to obtain baseline measures and monitor trends.15 In the United States, the COPD National Action Plan highlights the need to collect, analyze, report, and disseminate COPD-related public health data, and the need for more research to better understand the prevention, pathogenesis, diagnosis, treatment, and management of COPD.16
A registry is an ideal way to encourage increased and coordinated data collection, analyses, and data sharing. Registries enable us to observe the course of disease, to understand the impact of treatment variations and outcomes, and to examine factors that can influence prognosis.17 Registries are particularly suited to tracking the natural progression of COPD, in which progression is slow and trends can be obscure; describing care patterns and disparities in care delivery; and assessing effectiveness, safety, and quality of care.17 Prospective, observational patient registries have the advantages of including real-life patients (improving generalizability) and examining clinical questions for which a RCT is either impractical or unethical.
In response to the call to action of the COPD National Action Plan16 and the World Health Organization,15 the Advancing the Patient Experience in COPD (APEX-COPD) registry (https://www.apexcopd.org/) is the first primary care health system–based COPD registry in the United States.18,19 It was designed as an observational, primary care initiative which retrospectively and prospectively collects electronic health record (EHR) COPD variables (derived from larger data set—the COPD Optimum Patient Care Research DARTNet Research Database [COPD-RD]). And, unlike other registries, it links these EHR data to patient-reported information/outcomes (PRIO) data and information gathered from COPD patients during primary care visits.18 The APEX-COPD registry was established and co-funded by Optimum Patient Care Global and Boehringer Ingelheim. Optimum Patient Care Global retains intellectual property rights to the registry.
The primary objectives of the APEX-COPD registry are to describe the primary care COPD population in the United States; to compare the effectiveness of current COPD treatments; and to understand predictors of response to available COPD treatments.19 To improve COPD management in primary care, it is first necessary to describe the patient population using clinically relevant variables which can be collected practically and monitored longitudinally. The aim of the current study was to describe the demographic and clinical characteristics of COPD patients managed in US primary care using EHR and PRIO-matched variables (ie, APEX-COPD registry) and to assess the generalizability of these data against a large EHR-based COPD registry (ie, the COPD-RD).
METHODS
Design
This is a historical registry study using patient data from the COPD-RD from which the APEX-COPD registry is derived. The APEX-COPD registry contains all COPD-RD variables but also links these with PRIO data. The study was designed, implemented, and reported in accordance with relevant regulatory and ethical requirements (Supplemental Appendix).
Patients
Eligible patients from participating primary care sites had a diagnostic code for COPD (ICD-9-CM or ICD-10-CM) or a COPD monitoring review code (included chronic bronchitis, emphysema, alpha-1 antitrypsin deficiency, and mixed COPD/asthma) recorded in their EHR and were aged 35 or more years at the time of COPD diagnosis (Supplemental Table 1). These patients had current COPD defined as an appointment in the last 2 years and either had (1) been diagnosed or re-coded with COPD in the last year, (2) a prescription for a COPD inhaler in the last 2 years and a COPD diagnosis (including diagnoses with an end date), or (3) had active patient-reported COPD symptoms. Patients were excluded if they were participating in a COPD drug therapy clinical trial, expected to live less than 12 months, diagnosed with cancer in the past 3 years (excluding non-melanoma skin cancer), or were receiving hospice care.
Sites
Thirty-one clinic sites within 5 health care organizations (located in Texas, Ohio, Colorado, New York, and North Carolina) provided access to a limited EHR data set through a data use agreement of all patients with COPD who did not specifically opt out of the project.
Data Collection
Aggregated baseline demographic and clinical EHR data were collected from June 2019 through September 2020 and extracted remotely by the DARTNet Institute. PRIO data for the APEX-COPD registry were collected with a paper questionnaire or Patient Engaged Electronic Reporting System (a browser-based study management and PRIO data collection system), from December 2019 through November 2020 (Supplemental Appendix).
Study Variables
Demographic, clinical, and PRIO variables collected in the APEX-COPD registry were developed by Delphi consensus.18 They were subdivided into 3 types of variables: demographics, disease monitoring, and treatment (Supplemental Tables 2-4). Specific PRIO variables included the COPD Assessment Test, modified Medical Research Council (mMRC) dyspnea scale, and Global initiative for chronic Obstructive Lung Disease (GOLD) A-D groups, that describe COPD in terms of its impact on patients’ quality of life, severity of dyspnea, and symptom burden or risk of exacerbation, respectively (Table 1).20-22
Description of Variables Used for Disease Monitoring
Exacerbations were defined using EHR data (COPD-RD and APEX-COPD registry patients) and reported by patients themselves (APEX-COPD registry only). Exacerbation definition using EHR data was adapted from that published by Halpin and colleagues.23 Specifically, COPD exacerbations were defined according to a hierarchal algorithm as the occurrence of the following events: (1) exacerbation code (Supplemental Table 5); (2) COPD, acute bronchitis, lower respiratory tract infection, other lower respiratory code, or influenza code with prescribed oral corticosteroid or respiratory specific antibiotic or both; or (3) uncoded exacerbation with prescribed oral corticosteroid or respiratory-specific antibiotic or both (without other reason).
Patient-reported exacerbations were received from patient questionaires for those in the APEX-COPD registry. The full COPD exacerbation definition and the algorithm breakdown is provided in Supplemental Table 6. The EHR data look-back period is up to 10 years from September 2020. Medications and conditions (eg, diagnoses) variables, however, are not limited by a look-back period but extend back as far as EHRs exist at each site for patients included in the registry.
Statistical Analysis
Stata version 14 (StataCorp LLC) and R version 3.6 (R Project for Statistical Computing) were used to conduct all statistical analyses and data manipulations. Descriptive statistics were computed for all EHR demographic and clinical variables (both registries) and PRIO variables (APEX-COPD registry) as categorical variables and presented separately for the COPD-RD and APEX-COPD registry.
RESULTS
Demographic Characteristics
From June 2019 through November 2020, 17,192 eligible patients were included from the COPD-RD and 1,354 were includeed from the APEX-COPD registry (ie, had demographic, clinic, and PRIO data). From the 5 COPD-RD sites, there were 8,722 from Ohio, 6,038 from North Carolina, 1,149 from New York, 811 patients from Texas, and 472 from Colorado. Overall, patients were predominantly female (56%), aged 55-84 years old (81%), White (64%), smokers or ex-smokers (88%) and were overweight or obese (69%) (Table 2). We found no statistically significant difference between APEX-COPD and COPD-RD for race or ethnicity.
Demographics of COPD Patients in the COPD-RD and APEX-COPD Registry
Clinical Characteristics
Comorbidities
The comorbidity burden was high as 99% of COPD-RD patients had 1 comorbid condition, and polymorbidity was common with 87% of patients having 3 or more comorbidities. Hypertension was the predominant comorbidity (73%), followed by diabetes mellitus (45%), depression (42%), and osteoarthritis (41%) (Figure 1). Approximately one-third of patients also had gastro-esophageal reflux disease (39%), sleep apnea (37%), rhinitis (34%), asthma (33%), anxiety (31%), and anemia (31%) (Figure 1). More than 10% had pneumonia in the past year. Similar findings were noted in the APEX-COPD population, where 73% of patients had hypertension, 43% diabetes mellitus, 41% depression, and 41% osteoarthritis (Figure 1).
Prevalence of comorbidities in COPD patients in the COPD-RD (n = 17,192) and the APEX-COPD registry (n = 1,354).
APEX = Advancing the Patient Experience; COPD = chronic obstructive pulmonary disease; COPD-RD = COPD Optimum Patient Care DARTNet Research Database; CRS = chronic rhinosinusitis; GERD = gastresophageal reflux disease.
Exacerbations
Overall, 38% (6,579 of 17,192) of patients experienced 1 or more EHR-defined exacerbation in the past year (Table 3). Of these, 22% of patients experienced 1 exacerbation, 8% experienced 2, and 8% experienced 3 or more exacerbations in the past year (Figure 2). The APEX-COPD registry mirrored this finding with 47% of patients experiencing 1 or more exacerbations in the past year according to EHR and confirmed by patients (50% reported 1 or more exacerbation in the past year) (Table 4). The hierarchy of the exacerbation algorithms (Supplemental Table 6) shows the full exacerbation breakdown of frequencies at each defined subset.
Clinical Characteristics of COPD Patients in the COPD-RD and APEX-COPD Registry
Proportion of patients included in the COPD-RD (n = 17,192) and the APEX-COPD registry (n = 1,354) and who experienced exacerbations in the last year.
APEX = Advancing the Patient Experience; COPD = chronic obstructive pulmonary disease; COPD-RD = COPD Optimum Patient Care DARTNet Research Database.
Note: See Supplemental Table 6 for exacerbation algorithm.
Additional PRIO Data for COPD Patients in the APEX-COPD Registry (N = 1,354)
Treatment
In the last year, for COPD-RD patients, 3% were on no therapy, 9% were on reliever therapy only, and 88% were on controller therapy only (Figure 3; Table 5). For those on controller therapy, inhaled corticosteroid (ICS) with long-acting β2-agonist (LABA) was most commonly prescribed (30%), followed by ICS with LABA and long-acting muscarinic antagonist (LAMA) (27%), and LABA and LAMA (13%). ICS monotherapy was used by 4.% of patients. There were 47% of patients with any form of macrolide antiobiotic code in the last 12 months. APEX-COPD registry patients followed a similar pattern but with slightly more patients on no therapy (8%) or triple therapy (38%), and slightly fewer patients on reliever only (5%), ICS monotherapy (4%), or had any form of macrolide antibiotic (45%) compared with COPD-RD patients (Figure 3; Table 5).
COPD treatment use among patients included in the COPD-RD (n = 17,192) and APEX-COPD registry (n = 1,354).
APEX = Advancing the Patient Experience; COPD = chronic obstructive pulmonary disease; COPD-RD = COPD Optimum Patient Care DARTNet Research Database; ICS = inhaled corticosteroid; LABA = long-acting β2-agonist; LAMA = long-acting muscarinic antagonist; LTRA = leukotriene receptor antagonist.
a Prescribed with or without phopohodiesterase-4, macrolide, theophylline, or LTRA.
b Prescribed with LTRA.
c Prescribed with or without theophylline or LTRA.
Disease Management Therapy of COPD Patients in COPD-RD and APEX-COPD Registry
Blood Eosinophil Count
Mean steady state blood eosinophil count was 2,123 cells/µL in the COPD-RD population and 233 cells/µL in the APEX-COPD registry (Table 3), and was more than 300 cells/µL for 19% of COPD-RD patients and 22% of APEX-COPD registry patients.
PRIO Characteristics (APEX-COPD Registry Patients Only)
The majority of APEX-COPD patients reported a medium to very high COPD Assessment Test score (82%) and grade 1 or 2 mMRC-rated breathlessness (60%) (Table 4). However, 10% had a very high COPD Assessment Test score and 20% rated their breathlessness as grade 3 or 4 on the mMRC dyspnea scale. Although most patients reported no exacerbations (50%) and no hospitalizations (80%) in the past 12 months, 31% reported 2 or more exacerbations and 10% reported 2 or more hospitalizations (Table 4). The majority of patients, therefore, fell predominantly into GOLD B group (47%), being symptomatic but at low exacerbation risk. However, 35% of patients were in Group D (ie, symptomatic and at high exacerbation risk). More than 31% of APEX-COPD patients reported they continued to smoke.
DISCUSSION
To improve COPD management in primary care, it is first necessary to describe the patient population using clinically relevant variables which can be collected practically and monitored longitudinally. Our study is the first to do that, comprehensively describing US COPD patients managed in primary care. We described the COPD burden in a large primary care data set comprising EHR data from more than 17,000 patients representative of the general US primary care population and confirmed that the APEX-COPD registry is representative of this much larger data set. Furthermore, our EHR data gathered in the COPD-RD were enhanced with PRIO data from more than 1,000 patients enrolled in the APEX-COPD registry, providing greater insight into the burden of COPD on patients’ lives (eg, health status, breathlessness, and exacerbations). The APEX-COPD registry closes many COPD data gaps at the primary care level by encouraging increased and coordinated data collection, validation, analyses, as well as sharing and real-world application of data. This has been facilitated by the development of standardized data collection methods, use of harmonized definitions to monitor co-morbidity, symptom, and exacerbation burdens, as well as care and treatment of patients with COPD, using both EHR and PRIO data sources.18,19
The preponderance of smokers or ex-smokers, overweight or obese patients, and high prevalence of comorbidities in our study, and the almost equal distribution of males and females have been reported by others.24-28 Our prevalence of non-smokers (12% in both COPD-RD and APEX-COPD) is in agreement with that reported by Celli and colleagues (13%),29 but lower than that estimated by the Centers for Disease Control and Prevention (25%).30 It is hypothesized that in nonsmoking individuals COPD may be caused by abnormal lung development,31 passive smoking,32 or occur secondary to an auto-immune component.33 The comorbidity burden in COPD is historically high. It is important to accurately characterize COPD in primary care since it carries a high morbidity and socioeconomic burden, is associated with more hospitalizations, longer hospital stays, and more emergency department visits (unrelated to COPD).34-36 The cost of treating a COPD exacerbation is more than 4 times greater in patients with at least 1 comorbidity compared with patients lacking a co-morbidity.37 In agreement with other studies, comorbidities such as hypertension, diabetes mellitus, asthma, gastro-esophageal reflux disease, and psychological conditions (eg, anxiety and depression) were commonly reported by patients with COPD.3,34,35,38 The prevalence of these conditions varies markedly between studies, most likely influenced by differences in the populations studied (eg, smoking, age), medication interactions (eg, loop diuretic and β2-agonist; β-blocker and angiotensin-converting enzyme inhibitor), diagnosis coding accuracy, and lack of specific comorbidity case definitions.34 The results of our study are in good agreement with the most recent patient-reported US data that showed a self-reported prevalence of 61% with hypertension of, 41% with asthma, and 26% with diabetes mellitus.3 The global prevalence of asthma-COPD overlap among COPD patients has been estimated at 30% (from population-based studies).39
Exacerbation burden is equally important to define since COPD exacerbations have an impact on patient outcomes, mortality, and cost. Data from the National Inpatient Sample databases (2002-2010) showed that acute exacerbations of COPD-related hospitalizations accounted for 3% of all hospitalizations in 2010. Although both mortality and average length of stay decreased from 2002 to 2010, hospitalization cost increased from $22,187 in 2002 to $38,455 in 2010.40 Many studies have quantified COPD exacerbations from EHR data, using antibiotic and oral corticosteroid prescriptions and hospitalizations (among other criteria) to define exacerbations.23,28,41 Although these methods are well-validated and widely published, they do assume that patients use 1 prescription to treat 1 exacerbation. In the current study, exacerbations were defined using EHR criteria and reported directly by patients. We found good agreement between the percentage of patients with 1 or more exacerbation in the last 12 months (47% using EHR vs 50% reported by patients), similar to that previously reported in UK primary care.27,4
This exacerbation burden was somewhat surprising considering that 88% of these patients were on some form of maintenance therapy (predominantly ICS/LABA or ICS/LABA/LAMA), yet many continued to report high or very high CT scores and substantial levels of breathlessness (mMRC ≥2). These findings may indicate that (1) patients are not treated appropriately or are poorly compliant with their COPD medication regimens, (2) more focus on nonpharmacological treatment options is required to reduce symptom burden (eg, smoking cessation; more than 31% of patients continued to smoke), or (3) additional physician education is required to diagnose and treat COPD earlier to delay lung function decline.
An assessment of how well COPD responds to current treatments and the appropriateness of pharmacotherapy in these patients is the subject of future, planned research. Initial evidence of both over- and under-treatment, however, may be inferred from the current data. Over-treatment of some patients may be inferred from the fact that overall 27% of patients were on triple therapy (indicated for those with persistent exacerbations),1 and 30% were on ICS/LABA (recommended as an initial treatment for GOLD Group D patients, particularly for those with a blood eosinophil count of more than 300 cells/µL)1 even though only 16% experienced 2 or more exacerbations (in last 12 months) and 19% of patients had a blood eosinophil counts of more than 300. On the other hand, under treatment of some patients may be inferred from the fact that only 18% and 4% of APEX-COPD patients were on LABA/LAMA and LAMA, respectively. One may expect this would be higher considering that 45% of patients reported breathlessness (mMRC grade ≥2), but may also relate to the high cost of COPD inhalers in the United States. Furthermore, 4% of patients were on ICS monotherapy (not recommended), and 3% received no treatment.
It is important to recognize that these GOLD treatment recommendations are based on evidence from RCTs which, by virtue of their strict inclusion/exclusion criteria, may not be representative of many patients living with COPD in real-life.43 Taken together, these findings indicate further opportunity for medication optimization in the primary care setting for COPD patients and call for more real-life effectiveness trials, or broader inclusion criteria for RCTs to provide real-life evidence of response to and appropriateness of treatment and to inform COPD management guidelines.
Limitations of our study include the fact that registry data has lower internal validity than that collected prospectively in RCTs, and lacks information on treatment adherence, inhaler technique, and patient peak inspiratory flow. Low numbers of pneumococcal vaccinations were found in the EHR at one of the health care organizations due to completion data being stored externally in a state immunization registry. These limitations are counter balanced by the large data set of more than 17,000 primary care patients in the United States, standardization of data collected, and the fact that the APEX-COPD registry is representative of the huge COPD-RD data set, providing confidence in its generalizability to the US COPD population.
In conclusion, our findings highlight the high exacerbation, treatment, and comorbidity burdens experienced by US patients diagnosed with COPD and managed in primary care, and the need for more real-life effectiveness trials to better support decision making at the primary care level.
Acknowledgments
Kidane Gebremariam is acknowledged for his contribution to protocol development. We would also like to acknowledge Audrey Ang of the Observational and Pragmatic Research Institute, Singapore, for editorial and formatting assistance which supported the development of this publication.
Footnotes
Conflicts of interest: Pace is on the advisory board for Mylan; stock from Novo Nordisk, Pfizer, Novartis, Johnson and Johnson, Stryker, Amgen, Gilead, Sanofi.
Ratigan, Gaona, Kent, and Brandt are employees of the DARTNet Institute and report no conflicts of interest.
Carter, Evans, Stanley, and Li Voti are employees of Optimum Patient Care UK, a co-founder of the APEX-COPD initiative.
Chang and Fox declare no conflicts of interest.
Edwards, Kruszyk, and Le Lievre are employees of Optimum Patient Care Australia.
Han reports consulting for Boehringer Ingelheim, GlaxoSmithKline, and AstraZeneca, and research support from Novartis and Sunovion.
Kaplan is a member of the advisory board of, or speakers bureau for, AstraZeneca, Behring, Boehringer Ingelheim, Covis, Grifols, GlaxoSmithKline, Merck Frosst, Novo Nordisk, Novartis, Pfizer, Purdue, Sanofi, Teva, and Trudel.
Kocks declares grants and personal fees from AstraZeneca, Boehringer Ingelheim, Glaxo Smith Kline, and Novartis, and grants from Chiesi, Mundipharma, and Teva.
Mahle and Shaikh are employees of Boehringer Ingelheim, a co-founder of the APEX-COPD initiative.
Make reports funding from the NHLBI for the COPDGene study; grants and medical advisory boards from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, and Sunovian; personal fees for DSMB from Spiration and Shire/Baxalta;
CME personal fees from WebMD, National Jewish Health, American College of Chest Physicians, Projects in Knowledge, Hybrid Communications, SPIRE Learning, Ultimate Medical Academy, Catamount Medical, Eastern Pulmonary Society, Catamount Medical Communications Medscape, Eastern VA Medical Center, Academy Continued Healthcare Learning, and Mt. Sinai Medical Center; royalties from Up-To-Date; medical advisory boards from Novartis, Phillips, Third Pole, Science 24/7, and Vernoa; grants from Pearl.
Skolnik is on advisory boards for AstraZeneca, Teva, Lilly, Boehringer Ingelheim, Sanofi, Janssen Pharmaceuticals, Intarcia, Mylan, and GlaxoSmithKline; payment for lectures/speaking engagements from AstraZeneca and Boehringer Ingelheim; research support from Sanofi, AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline.
Yawn has served on COPD-related advisory boards for GlaxoSmithKline, AstraZeneca, Novartis, and Boehringer Ingelheim, and received COPD-related investigator-initiated research funds from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, and Novartis.
Price has advisory board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Thermofisher; consultancy agreements with Airway Vista Secretariat, AstraZeneca, Boehringer Ingelheim, Chiesi, EPG Communication Holdings Ltd, FIECON Ltd, Fieldwork International, GlaxoSmithKline, Mylan, Mundipharma, Novartis, OM Pharma SA, PeerVoice, Phadia AB, Spirosure Inc, Strategic North Limited, Synapse Research Management Partners S.L., Talos Health Solutions, Theravance, and WebMD Global LLC; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Theravance, and UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals, and Sanofi Genzyme; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis, Thermofisher; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation program, and Health Technology Assessment; and was an expert witness for GlaxoSmithKline.
- Received for publication March 21, 2021.
- Revision received December 13, 2021.
- Accepted for publication January 6, 2022.
- © 2022 Annals of Family Medicine, Inc.