Abstract
PURPOSE Unlike in many community-based settings, benzodiazepine (BZD) prescribing to older veterans has decreased. We sought to identify health care system strategies associated with greater facility-level reductions in BZD prescribing to older adults.
METHODS We completed an explanatory sequential mixed methods study of health care facilities in the Veterans Health Administration (N = 140). Among veterans aged ≥75 years receiving long-term BZD treatment, we stratified facilities into relatively high and low performance on the basis of the reduction in average daily dose of prescribed BZD from October 1, 2015 to June 30, 2017. We then interviewed key facility informants (n = 21) who led local BZD reduction efforts (champions), representing 11 high-performing and 6 low-performing facilities.
RESULTS Across all facilities, the age-adjusted facility-level average daily dose in October 2015 began at 1.34 lorazepam-equivalent mg/d (SD 0.17); the average rate of decrease was −0.27 mg/d (SD 0.09) per year. All facilities interviewed, regardless of performance, used passive strategies primarily consisting of education regarding appropriate prescribing, alternatives, and identifying potential patients for discontinuation. In contrast, champions at high-performing facilities described leveraging ≥1 active strategies that included individualized recommendations, administrative barriers to prescribing, and performance measures to incentivize clinicians.
CONCLUSIONS Initiatives to reduce BZD prescribing to older adults that are primarily limited to passive strategies, such as education and patient identification, might have limited success. Clinicians might benefit from additional recommendations, support, and incentives to modify prescribing practices.
INTRODUCTION
The potential safety risks of benzodiazepine (BZD) use among older adults have been known for more than 30 years.1,2 Whereas a variety of interventions have shown efficacy in reducing BZD prescribing—identified in a recent review as related to patient-related matters (eg, education regarding risks and benefits), prescriber skills and awareness (eg, clinical decision support), and health system constraints (eg, policy limits on prescribed supply)3—BZD use in the United States has remained remarkably steady.4 There are few examples of successful real-world initiatives that have reduced BZD prescribing in the United States. Therefore, the reduced rate of BZD prescriptions for older adults in the US Department of Veterans Affairs (VA) system, decreasing by nearly one-half from 2013 to 2017,5 is notable.
During that time, BZD prescribing to high-risk populations was a focus of the VA Psychotropic Drug Safety Initiative (PDSI), which launched in 2013. The PDSI is a nationwide psychopharmacology quality improvement (QI) initiative to improve the safety and effectiveness of psychopharmacologic treatment.6 Whereas the PDSI program goals are centrally planned and disseminated, each individual medical center designs and implements its own QI strategies. Phase 2 of the PDSI, which ran from October 2015 to June 2017, focused specifically on prescribing to older veterans; among the QI target options, approximately one-third of the facilities specifically selected to prioritize reducion of BZD prescribing (hereafter referred to as “priority facilities”).
Given the continued widespread BZD prescribing in the community, the VA provides a stark contrast. This explanatory sequential mixed methods study was designed to study real-world BZD reduction strategies implemented in facilities within the VA, the country’s largest integrated health care system. We began with a quantitative phase to sort facilities by BZD reduction outcomes, hypothesizing that priority facilities (ie, those that specifically targeted BZD prescribing as a part of PDSI phase 2) would experience greater reductions in BZD prescribing. To place the quantitative findings into context, we then transitioned to a qualitative phase to explore BZD reduction strategies that facilities implemented locally.
METHODS
This study was approved by the VA Ann Arbor Institutional Review Board, which provided a waiver of written consent. We used an explanatory sequential mixed methods design, in which quantitative analyses of facility-level BZD prescribing were used to identify facilities for qualitative interviews to better understand local strategies used to address BZD prescribing. This included data integration via connecting (ie, qualitative sampling based on quantitative facility performance) and merging (ie, qualitative findings [strategies] interpreted in light of quantitative performance [BZD reduction]).7 We also used data integration during analysis via use of a joint display, which is a simultaneous array of aspects of the quantitative and qualitative data.7,8
Quantitative Sample and Analysis
Using the Veterans Health Administration Corporate Data Warehouse, the central data repository derived from the VA’s systemwide electronic health record (EHR), we constructed a cohort of long-term BZD users to examine change in BZD prescribing during PDSI phase 2 (ie, the 7 quarters from October 1, 2015 to June 30, 2017). First, we identified veterans aged ≥75 years who were active long-term BZD users at the start of phase 2, defined as having >120 days’ BZD supply during the prior 12 months.9 To ensure that we included active long-term users at the start of phase 2, we further limited to those with ≥1 BZD prescription filled in the 90 days before October 2015. We excluded patients in an inpatient or long-term care setting for the entire phase, as well as those who received hospice care or died.
Beginning with the quarter before the start of phase 2 and continuing through the 7-quarter timeframe, we used prescription fill date, days’ supply, quantity, and drug name from VA outpatient prescription fills to calculate the average daily dose of each patient’s BZD exposure in oral lorazepam-equivalent mg/d.10 Each quarter, veterans were assigned to a facility on the basis of the location of their primary care physician.
We modeled facility-level average daily dose of BZDs over time among long-term BZD users and included terms for whether the facility was a priority facility (ie, chose to focus on BZD prescribing) during phase 2 (ie, an indicator if the facility did [1] or did not [0] prioritize BZD reduction), quarter (Q0-Q6 [eg, Q0 represents the quarter from October 1, 2015 to December 31, 2015]), their interaction, and facility-level average age (centered). Facility-level random intercepts and slopes were included to allow for facility variation in the outcome at the start of the phase and over time. Model results, including fixed and random effects estimates, were used to estimate the expected facility-level average daily dose of BZDs at the start of the phase and over time (slope), with facility-level average age set to the mean. The interaction between being a priority facility and time allowed us to examine if change in facility-level average daily BZD dose over time differed by priority status. The α level for statistical significance was set at .05, and the test was 2-sided.
Qualitative Sample, Data Collection, and Analysis
We used facility estimates to rank facilities by change in BZD prescribing over time (ie, slope from the quantitative model [yearly change in average daily dose at the facility level]) and then select high- and low-performing facilities (ie, those with larger and smaller average dosage changes, respectively) for qualitative interviews. In addition to selecting facilities within performance strata, we considered facility size (classified as small, medium, and large on the basis of the number of older veterans receiving care) and geography (US Census region) to ensure a diverse sample of facilities (eg, not entirely large or southern facilities). The original intent was to select 6 high and 6 low performers from among the BZD priority facilities. However, counter to our original hypothesis, several facilities that achieved significant BZD reductions during phase 2 were nonpriority facilities (ie, they did not prioritize BZD prescribing and focused on other non-BZD quality measures). We expanded the sampling frame to include 5 nonpriority but high-performing facilities.
We conducted telephone interviews with each site’s designated PDSI champion (or cochampions at some sites), as selected by the site and reported to the national PDSI program. Interviewees were recruited primarily by e-mail, with telephone follow-up as needed. Two study team members, a clinician (D.T.M.) and a qualitative methodologist (L.T.), conducted the interviews, which lasted 30 to 60 minutes. The interviews began with brief introductions, interviewer backgrounds, and an overview of the study purpose. The interviews were then conducted using a semistructured guide, which covered site strategies to reduce BZD prescribing; how strategies were selected; personnel involved in strategy delivery; and strategy barriers or facilitators (Supplemental Appendix). A team member took notes during each interview and added verbatim quotes after reviewing the digital audio recording of the interview.
Given the focused nature of the evaluation, we used a rapid analysis approach to identify and characterize specific strategies for reducing BZD prescribing.11,12 We developed a summary template for recording information within key domains, following the general structure of the interview guide, focusing on the role of each champion, site strategies, and organizational dynamics. Two study team members (D.T.M. and L.T.) then used data from the interview notes to independently create summaries for each interview according to these domains, which were reconciled via discussion. Summary data were subsequently transferred to a matrix to create site composites, allowing for further interpretation and comparison across sites.13-15 The matrix was reviewed by the entire multidisciplinary research team, and findings were developed via discussion during team meetings.
RESULTS
Across the VA, we identified 24,512 veterans aged ≥75 years who met the definition of long-term BZD use at the start of PDSI phase 2, comprising 3.0% of all older veterans with an outpatient prescription and 58.1% of older BZD users at that time. Select cohort characteristics are presented in Table 1. Across the 140 facilities, the median number of veterans aged ≥75 years prescribed long-term BZD at the start of phase 2 was 148 (Q1-Q3, 109-222.5). Overall during phase 2, the average dose of BZD prescribed to this group decreased at an annualized rate of −0.27 mg/d (SD 0.09; Table 2). At the 47 facilities that prioritized reducing BZD prescribing, the rate of annual decrease was −0.29 mg/d (SD 0.09) compared to −0.26 mg/d (SD 0.09) at nonpriority facilities. The rate of decrease between priority and nonpriority facilities was not significantly different (facility priority status × time interaction P = .07).
Demographic and Clinical Characteristics of Chronic BZD Users at the Start of PDSI Phase 2
Facility-Level Average BZD Use at the Start of PDSI Phase 2 and Expected Quarterly Change Among a Cohort of Long-Term BZD Users, Overall and By Facility Priority Status
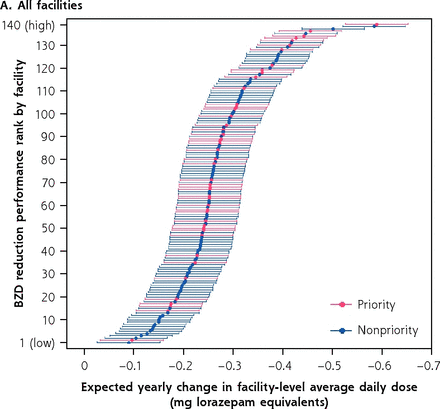
Figure 1a shows all 140 facilities ranked by annual decrease in BZD dose. Figure 1b shows the priority (low- and high-performing; n = 11 total) and high-performing nonpriority (n = 5) sites that participated in semistructured telephone interviews, which were completed from July 2019 to November 2019. Select facility characteristics are presented in Table 3. Because some sites identified cochampions, we interviewed a total of 21 individuals (14 clinical pharmacists, 4 psychiatrists, 1 primary care physician, 1 physician assistant, 1 nurse).
Expected yearly change in facility-level average daily BZD dose among a cohort of long-term BZD users.a,b
BZD = benzodiazepine.
a Priority and nonpriority refer to whether, as part of Psychotropic Drug Safety Initiative phase 2, facility chose to focus on BZD deprescribing to older veterans.
b High-performing facilities achieved a greater change in average daily dose of BZD prescribing; low-performing facilities, lesser.
Average Annual Change in Facility BZD Prescribing and Facility Strategies to Reduce Prescribing
Whereas we initially hypothesized that there would be meaningful strategy differences by facility priority status, given the nonsignificant findings in the quantitative analysis, we did not use priority status to stratify our qualitative analysis. Rather, on the basis of the themes that emerged across facility interviews, we sorted strategies as passive or active.18 Passive strategies generally focused on enhancing knowledge but were potentially easy for clinicians and patients to disregard. Active strategies were designed to counter prescribing inertia—a common barrier to deprescribing19—and encourage clinicians to either deprescribe BZDs or provide an explicit clinical rationale for why continued prescribing was appropriate. As shown in Table 3 and described below, whereas passive strategies were commonly used across all facilities, active strategies were more often found in high-performing facilities.
Passive Strategies
Identifying Hot Spots
This strategy included identifying priority patients, clinicians, or clinics for targeted focus. The majority of sites used ≥1 of these strategies (3 of 6 low-performing [LP] sites, 7 of 10 high-performing [HP] sites). Some sites used the PDSI patient dashboard available at every facility to identify patients aged ≥75 years prescribed a BZD and provided a list of priority patients to clinicians. Other facilities modified or expanded the PDSI measure to identify patients coprescribed both a BZD and an opioid (eg, sites C and Y), or they lowered the age to patients aged ≥65 years prescribed a BZD (eg, sites B and X). Other facilities identified specific clinicians with large numbers of target patients.
Providing Patient- and Provider-Facing Education
The most common way that sites provided education was to have the champion or an academic detailer (ie, a specific VA pharmacy role providing unbiased education to support clinical decision making) present information on the potential risks of BZD treatment in older adults, alternative drugs for common indications (eg, insomnia, anxiety), tapering methods, and facility services to which clinicians could refer patients. This information was typically presented at psychiatry or primary care monthly staff meetings, grand rounds, or “lunch and learns.” Such provider-facing education occurred at 5 LP and 7 HP sites. Several sites also provided content, including 3 sites that created “difficult conversation aids,” to help clinicians with the perceived challenge of convincing patients to reduce BZDs.
Patient-facing education, most typically the VA-modified version of the Eliminating Medications Through Patient Ownership of End Results (EMPOWER) tool,20 was also commonly used at 3 LP and 6 HP sites.
Providing Services and Tools
A passive strategy offered by 4 HP sites was to provide tools, generally integrated into the EHR, to help clinicians implement a BZD taper. Some facilities offered provider tapering support, either face-to-face or via EHR review by a consulting clinician such as a clinical pharmacist (2 LP, 5 HP sites). Three HP sites allowed clinicians to refer patients to a specialist for the taper, whereas 1 LP and 2 HP sites established a designated taper clinic, which offered a permanent service rather than ad hoc consultation. Scope of practice in a facility’s state determined whether a clinical pharmacist could carry out a taper or provide recommendations to the primary provider.
Active Strategies
Providing Guidance
Several initiatives entailed providing guidance, which we considered to go beyond a passive strategy because these facilities targeted specific patients or clinicians and included potentially actionable recommendations.
At 2 HP sites (sites V and X), the site champion performed chart reviews and sent personalized letters to patients. At site V, an EMPOWER brochure was sent in advance of an upcoming appointment with the BZD-prescribing clinician. The champion included a cover letter, often including a personalized note, that asked patients to read the brochure and bring it to their next visit. At site X, the champion drafted a letter for patients that was reviewed, signed, and sent, along with an EMPOWER brochure, on behalf of the prescribing clinician. The letter explained why the patient should be tapered off their prescribed BZD and encouraged them to discuss potential alternatives with the prescriber. The champion also sent an e-mail to the prescriber with some respectful suggestions for alternate management strategies.
Some facilities implemented EHR order sets to provide treatment options and guide clinicians’ actions. As implemented, clinicians could not prescribe a BZD without reviewing the alternative treatment options suggested (eg, site Z provided orders for alternative pharmacotherapy or specialist referral). Some sites (eg, site Q) also created a BZD quick order set for tapering (ie, a templated set of orders in the EHR that reduced the baseline dose by a standard percentage per time interval).
Several facilities also used guidance strategies with an additional degree of action. For example, 1 LP and 1 HP facility (sites D and Q) offered patient groups focused on non-pharmacologic anxiety management. We considered patient groups to be an active strategy because ongoing contact with patients could provide emotional support during a difficult process, yield benefit from peer support, and alert clinicians if patients were having difficulties.
Facilities also offered guidance to clinicians even further on the active continuum, including 1-on-1 meetings by champions with clinicians with high levels of BZD prescribing (1 LP, 6 HP). At 1 HP site (site R), clinical pharmacists monitored taper progress and sent clinicians a reminder if there had been no progress in 90 days.
Barriers to Prescribing
Several facilities, including the majority of HP sites, implemented administrative barriers to BZD prescribing (1 LP, 7 HP). For example, 3 sites required a consultation when a new BZD was prescribed. In these facilities, the clinician could prescribe a short initial supply (ie, 7 days) but was then required to complete a case review with a clinical pharmacist or the PDSI champion before prescribing additional days. Other barriers included new documentation requirements for ongoing BZD prescriptions, a urine drug screen, or a face-to-face appointment with patients for a prescription. Site Y required that if no dose reduction occurred over a period of 90 days for patients being tapered, the clinician complete a case review with the service chief.
Performance Measures
A limited number of facilities incorporated BZD prescribing into clinician performance measures (1 LP, 3 HP). One LP site (site E) considered prescribing to older veterans in physicians’ annual reviews, whereas it was part of credential renewal at an HP site (site R). At site R, BZD prescribing was reviewed at clinicians’ quarterly reviews with their service chief. The chief noted, “[performance in tapering patients BZDs] was always brought up as an option like, ‘We can talk about this if you have questions about your dashboard,’ with the implication that I know how they’re doing that if they’re having struggles, then they need to be proactive about their prescribing practice and get support.” At that site as well as 1 other HP site (site Q), a BZD performance metric was part of clinicians’ pay for performance.
Personal Authority
Champions at 3 HP sites exhibited exceptional personal authority or leadership. At 2 sites (sites V and X), the champions had >20 years of experience at their respective facilities, were highly regarded by their peers, and had developed strong relationships throughout their facility. When asked how much site X’s success was attributable to the champion, a provider answered, “A hundred percent, well let’s say 80%, 100% that she did the teaching, and then the rest of the work was done by the clinicians, but she [was] the guiding person.” The site X champion did individual chart reviews—having one’s charts reviewed by a senior and highly respected individual likely conveyed that BZD reduction was taken seriously at that facility. At site V, the champion worked 1-on-1 with clinicians; she used motivational interviewing techniques to assess clinicians’ needs, brainstorm how to overcome barriers, and get a statement of commitment. Finally, at site R, no strategies included hard stops to stop clinicians’ BZD prescribing. However, the site R champion had a facility leadership role and was willing to use personal authority to drive change, “…[clinicians] could still conceivably have gotten [BZDs] if they dared to risk the wrath of me.”
DISCUSSION
Whereas BZD prescribing to older adults is appropriate for select indications,21 the prevalence of use almost certainly exceeds that.22 Unlike in community settings, BZD prescribing to older adults treated by the VA has been decreasing since 2013 and might therefore offer useful lessons for other settings working to improve the appropriateness of prescribing.5 The absolute size of the BZD decreases were relatively small; however, they are still meaningful because there is a dose-response relation between BZDs and their associated side effects and harms.23-25
The present research study was designed with the hypothesis that the priority vs nonpriority distinction would be associated with facility-level differences in reducing the amount of BZD prescribing. Counter to our hypothesis, several nonpriority facilities reduced BZD prescribing similarly to the highest-performing priority facilities. Therefore, when selecting facilities for the qualitative phase to explore BZD reduction strategies and place these quantitative findings into context, we chose to include several high-performing but nonpriority facilities. We learned that these facilities—which were nonpriority by virtue of selecting alternative PDSI QI targets (eg, reducing anticholinergic medications)—had focused on reducing opioid-BZD coprescribing via the VA’s nationwide Opioid Safety Initiative.26 These facilities’ strategies were consistent with those of facilities exclusively focused on BZDs but were often presented as part of an either/or approach, in which the patient could choose which drug to discontinue. Given the similarities, we retained these nonpriority facilities in the qualitative analysis.
Using qualitative analysis, we arrived at the passive vs active strategy distinction as opposed to priority vs non-priority status to help understand performance differences. The single strategy used by all facilities was to provide information, a relatively passive strategy. For several facilities, this took the form of patient-facing education, typically use of the EMPOWER brochure adapted for use in the VA. Provider-facing education focused on informing clinicians of safety risks of BZD prescribing to older adults and providing information on alternatives, as well as directing clinicians to a facility dashboard identifying older veterans prescribed BZDs. Facilities with few additional strategies were generally low performers; this is not surprising given the inertia of BZD prescribing in the community, which has not changed despite decades of information about potential harms.1,2
In contrast, high-performing facilities used more active strategies—external processes designed to facilitate deprescribing or make continued prescribing more difficult. Some provided guidance to prescribing clinicians, often via the EHR (eg, suggesting alternative drugs or BZD tapering algorithms). At other facilities, guidance was provided by consulting clinicians via chart reviews or referral clinics (eg, clinical pharmacy tapering service). While varying in specific form, these services went beyond simply providing knowledge by prompting prescribing change with suggested next steps. Facilities that also used barriers to prescribing likely enhanced the effect of this guidance by creating resistance to the inertia of continuing the BZD prescription, which is known to limit deprescribing.19
The remaining active strategies—performance measures and personal authority—increased clinicians’ motivation to change their prescribing behavior.27 For example, BZD-related performance measures factored into clinician annual bonus structure or credentialing, both of which provided an external incentive to motivate clinical decision making. Finally—the strategy most difficult to consistently replicate—there was the personal authority of clinicians at several facilities. That authority by itself did not lead to reduced BZD prescribing; rather, the authority of those champions likely moderated the effects of other strategies.
Our mixed methods analysis has several limitations. First, because this was not a randomized controlled trial, we cannot attribute facility performance to the specific strategies that were used. Our quantitative analysis did not account for BZDs obtained from community sources; therefore, the decreases observed might not reflect true decreases in BZD exposure. In addition, our analysis did not consider BZD prescribing in long-term care settings. Our qualitative data were based on interviews with designated site champions and might not fully reflect facility strategies as they were experienced by frontline staff. Finally, it is unclear the extent to which these findings are generalizable to non-VA health systems or to younger patients, although the general strategies would likely apply.
CONCLUSION
The real-world strategies outlined here are consistent with those identified in reviews of interventions to reduce BZD prescribing3,28 and also align with findings from a recent commentary on using principles of deimplementation to advance deprescribing.29 Those authors classified deprescribing interventions using the Capability, Opportunity, Motivation, Behavior (COM-B) model of behavior, which identifies 3 factors that must be present for a behavior to occur—capability, opportunity, motivation—and found that while most interventions focused on only 1 element, success likely depends on addressing all 3. Indeed, we found that the high-performing facilities in the present analysis generally did, addressing capability (eg, patient- and provider-facing education), opportunity (eg, taper consultation), and motivation (eg, performance measures). For other health care systems that choose to address BZD deprescribing, this is important evidence suggesting that multicomponent interventions are necessary to support the difficult work of patient and clinician behavior change.
Footnotes
Conflicts of interest: Dr Wiechers is National Director of the VA Psychotropic Drug Safety Initiative. Other authors report none.
Funding source: Merit Award Number I01HX002340 (IIR 16-210) from the US Department of Veterans Affairs Health Services R&D Service.
- Received for publication May 18, 2021.
- Revision received January 11, 2022.
- Accepted for publication March 1, 2022.
- © 2022 Annals of Family Medicine, Inc.