Abstract
PURPOSE COVID-19 is a condition that can lead to other chronic conditions. These conditions are frequently diagnosed in the primary care setting. We used a novel primary care registry to quantify the burden of post-COVID conditions among adult patients with a COVID-19 diagnosis across the United States.
METHODS We used the American Family Cohort, a national primary care registry, to identify study patients. After propensity score matching, we assessed the prevalence of 17 condition categories individually and cumulatively, comparing patients having COVID-19 in 2020-2021 with (1) historical control patients having influenza-like illness in 2018 and (2) contemporaneous control patients seen for wellness or preventive visits in 2020-2021.
RESULTS We identified 28,215 patients with a COVID-19 diagnosis and 235,953 historical control patients with influenza-like illness. The COVID-19 group had higher prevalences of breathing difficulties (4.2% vs 1.9%), type 2 diabetes (12.0% vs 10.2%), fatigue (3.9% vs 2.2%), and sleep disturbances (3.5% vs 2.4%). There were no differences, however, in the postdiagnosis monthly trend in cumulative morbidity between the COVID-19 patients (trend = 0.026; 95% CI, 0.025-0.027) and the patients with influenza-like illness (trend = 0.026; 95% CI, 0.023-0.027). Relative to contemporaneous wellness control patients, COVID-19 patients had higher prevalences of breathing difficulties and type 2 diabetes.
CONCLUSIONS Our findings show a moderate burden of post-COVID conditions in primary care, including breathing difficulties, fatigue, and sleep disturbances. Based on clinical registry data, the prevalence of post-COVID conditions in primary care practices is lower than that reported in subspecialty and hospital settings.
- long COVID
- post-COVID conditions
- post-infectious disorders
- primary health care
- family practice
- chronic illness
- morbidity
INTRODUCTION
The direct and immediate impact of COVID-19 on the health of the US population has been of generational significance, and the secondary wave of persistent symptoms and new conditions could represent an equally substantial burden on the health of the US population. As of May 2023, approximately 104 million Americans were known to have been infected with SARS-CoV-2.1,2 Most affected individuals experience mild to moderate symptoms.3 Although greater severity of COVID-19 illness and hospitalization may increase the risk of post-COVID conditions (PCC), also known as long COVID, individuals with mild to moderate COVID-19 presentations may develop persistent symptoms as well.3 Estimates of PCC at 6 months after infection range from 20% to 54%,4-7 which translates to 19 to 51 million Americans having experienced this condition. Even more conservative estimates of 7.7 to 23 million affected Americans still represent a substantial public health problem.8
Research describing the clinical manifestations of PCC is mounting,9-13 yet there are limitations to existing evidence, in particular with respect to health care delivery needs in the United States. Nasserie et al14 identified several issues with existing PCC studies: limited generalizability due to patient selection criteria (eg, the tendency to enroll hospitalized patients); lack of variability in patient characteristics (eg, limited age ranges, unknown underlying health statuses); insufficient follow-up time; and variability in the outcomes measured. Furthermore, existing studies tend to be prospective and measure the prevalence of symptoms preselected by researchers. Although a prospective study design is highly useful for identifying possible clinical manifestations, the symptoms associated with PCC may not be severe enough to drive individuals in the general population to seek health care or to warrant diagnosis by a clinician. In addition, patients treated in primary care settings in the United States are largely missing from the PCC literature.
We sought to quantify cumulative morbidity for patients with COVID-19 before and after their infection in the primary care setting using propensity score–matched control patients. We looked at temporal trends in conditions individually and combined, following patients longitudinally from 1 year before to 6 months after an initial COVID-19 diagnosis.
METHODS
Data Source
The American Family Cohort is a data set derived from the American Board of Family Medicine PRIME Registry, an outpatient clinical data registry of primary care practices established in 2016. We used electronic health record data from this cohort beginning January 1, 2017, and ending March 31, 2022, capturing data on 3.9 million patients residing in all 50 states who had 32.2 million patient visits.
Patient Groups
COVID-19 Cases
We identified COVID-19 using the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code U07.1. COVID-19 cases were patients given this diagnosis during April 1, 2020 through October 2, 2021. Diagnosis date was defined as the date of the first instance of the COVID-19 diagnosis code during a primary care visit. Patients were eligible for inclusion if they were aged 18 years or older at diagnosis, had at least 1 primary care visit more than 365 days before diagnosis, and had at least 1 visit 14 to 365 days before diagnosis. We excluded patients who became inactive within 180 days after their diagnosis date because their primary care practices became inactive (stopped reporting data to the registry), which affected patient follow-up.
Historical Control Patients
Historical control patients had a diagnosis of influenza-like illness (ILI) between January 1, 2018 and December 31, 2018. We identified ILI using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM), ICD-10-CM, and Systematized Nomenclature of Medicine (SNOMED) codes (Supplemental Table 1). Diagnosis date was defined as the date of the first visit with an ILI diagnosis code in 2018. Age and visit inclusion criteria were the same as those for the COVID-19 cases described above. To minimize misclassification, patients who met the inclusion criteria to be historical controls were excluded from the COVID-19 cases for analyses involving historical controls.
Contemporaneous Control Patients
Contemporaneous control patients were identified for the years 2020 (April 1-December 31) and 2021 (January 1-October 2). This cohort was separated into 2020 and 2021, respectively. Our rationale for splitting the cohort was twofold: (1) in 2020, patients would not have been vaccinated against COVID-19, and (2) COVID-19 mitigation and prevention policies (eg, lockdowns, masking), as well as health care use, may have differed across years. The latter would affect the identification of PCC. Patients in these cohorts had a wellness or preventive care visit during the corresponding calendar year, identified using Current Procedural Terminology codes (Supplemental Table 2). Date of inclusion was defined as the date of the first visit with a wellness or preventive visit code during each calendar year. We applied the same inclusion and exclusion criteria as described for historical control patients.
Outcomes
Through expert consensus, we identified 17 diagnostic categories that capture the range of symptoms and conditions experienced by patients after acute COVID-19 illness; across all 17 categories, we selected ICD-10-CM codes expected to appear in the patient’s electronic health record if the patient was being treated for the symptom or condition (Supplemental Table 3).13,15,16 Patients who received care for any of the conditions within a diagnostic category after their diagnosis/inclusion date were considered to have a postdiagnosis condition within that category. For the historical comparison, we also assessed if patients had been treated for any of the conditions 14 to 365 days before their diagnosis date; if so, we considered them to have a preexisting condition within the diagnostic category. We included each of these conditions in our tally of total conditions.
Each diagnostic category was considered an individual component of composite cumulative morbidity for the patient. We created a cumulative morbidity indicator ranging from 0 to 17 that sums the number of diagnostic categories in which the patient had a diagnosis. For example, a patient with 3 ICD codes across 2 diagnostic categories was considered to have a composite cumulative morbidity of 2. As patients accumulated evidence of new diagnostic categories during follow-up, their cumulative morbidity increased. All 17 diagnostic categories were considered chronic conditions for the entirety of the study period; therefore, patients determined to have any individual condition at a certain point in time would retain that condition throughout their follow-up.
Statistical Analysis
To account for selection bias and to estimate the association between COVID-19 and potential PCC, we used propensity score matching. We conducted propensity score–matched analyses to quantify the incremental risk of the 17 diagnostic categories/conditions between COVID-19 patients and the 2 comparator cohorts: (1) historical control patients with an ILI diagnosis in 2018, and (2) contemporaneous control patients who had wellness or preventive visits in each of 2020 and 2021, respectively.
For all propensity scores, we used 1:1 caliper matching without replacement with a caliper of 0.01. Propensity scores were calculated using multivariate logistic regression analysis to estimate a patient’s propensity to being assigned to the cases group (COVID-19), accounting for observable characteristics: age, gender, race and ethnicity, social deprivation index, prediagnosis/preinclusion health care use evidenced by primary care visits, and prediagnosis/preinclusion morbidity within the 17 diagnostic categories. For analyses using contemporaneous control patients, we also matched groups on the year and month of diagnosis/inclusion. All matching variables, aside from dates, were categorical variables. We performed the matching process and the analyses described below using age as a continuous variable as well for sensitivity purposes. To assess the balance of prematched and post-matched covariates across analyses, we evaluated the absolute difference in prevalence across groups as well as the Cohen h to assess clinical meaningfulness in descriptive statistics. General guidelines for interpreting the Cohen h suggest that a value of 0.20 is considered the threshold for a small effect, 0.50 for a medium effect, and 0.80 for a large effect.17-19
To compare the various diagnostic categories/conditions between patients with COVID-19 and historical control patients with ILI, we conducted a primary analysis estimating prediagnosis and postdiagnosis trends in cumulative morbidity with an interrupted time series analysis using generalized estimating equations with repeated measures on patients (see Supplemental Appendix for a description of this method). A secondary analysis was performed using a restricted cohort of only those COVID-19 and ILI patients who had at least 1 primary care visit 180 days or more after their diagnosis. This requirement increased certainty that the patients remained active in the practice for at least 6 months after their diagnosis and that any subsequent conditions requiring primary care attention were captured for these patients.
To compare the various diagnostic categories/conditions between patients with COVID-19 and contemporaneous control patients attending a wellness or preventive visit, we first separated the cohort by calendar year. We matched patients with COVID-19 diagnosed during April 1, 2020 through December 31, 2020 to control patients who had a visit in the same month of that year and compared absolute differences in proportions of diagnostic categories during the postdiagnosis period as a one-time, cross-sectional snapshot. We completed the same process for patients with COVID-19 diagnosed during January 1, 2021 through October 2, 2021.
RESULTS
Comparison With Historical Controls
We identified 52,889 patients with a COVID-19 diagnosis from April 1, 2020 through October 2, 2021, of whom 28,215 met the criteria for study inclusion (Table 1). There were 423,223 patients with an ILI diagnosis in 2018, of whom 235,953 met the inclusion criteria to serve as historical controls. After matching, we retained 27,960 patients with COVID-19 and 27,960 control patients with ILI in the primary analysis. For the secondary analysis using a restricted sample of patients with a primary care visit 180 days or more after their diagnosis, we matched 14,805 patients with COVID-19 to 14,805 control patients with ILI.
Prematched Characteristics of Patients With COVID-19 and Historical Control Patients With ILI
After diagnosis, the most prevalent diagnostic categories/conditions for patients with COVID-19 were consistent across all analyses regardless of the type of control patient (historical or contemporaneous) (Figure 1). Breathing difficulties, fatigue, type 2 diabetes, and psychological conditions predominated.
Prevalence of diagnostic categories in the matched samples after diagnosis or inclusion.
For the primary analysis with historical ILI control patients, patients with COVID-19 had a slightly higher postdiagnosis prevalence of breathing difficulty (4.4% vs 3.2%) (Figure 1A). Differences in postdiagnosis morbidity were slightly greater in the secondary analysis: patients with COVID-19 had a higher postdiagnosis prevalence of breathing difficulties (4.2% vs 1.9%), type 2 diabetes (12.0% vs 10.2%), fatigue (3.9% vs 2.2%), and sleep disturbances (3.5% vs 2.4%). All differences were less than 3%, with most less than 1%, and all effect size estimates were less than 0.20, suggesting little or no meaningful difference.
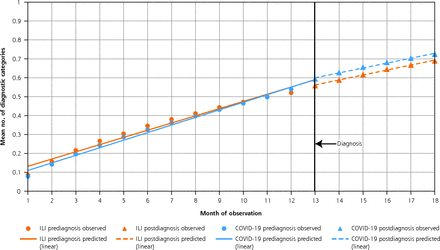
In the primary analysis, patients with COVID-19 and patients with ILI had a similar mean number of other diagnoses/conditions at diagnosis, 0.52 and 0.54, respectively (Figure 2). In the 6 months after diagnosis, patients with COVID-19 experienced monthly increases in cumulative morbidity (trend = 0.026; 95% CI, 0.025-0.027; P <.001) equivalent to those in ILI control patients (trend = 0.026; 95% CI, 0.023-0.027; P <.001). Relative to their respective prediagnosis slopes, slopes in cumulative morbidity after diagnosis for both groups differed; patients with COVID-19 had 0.014 fewer (95% CI, −0.015 to −0.013; P <.001) new diagnoses per month after diagnosis as compared with before diagnosis, while patients with ILI had 0.012 fewer (95% CI, −0.013 to −0.011; P <.001). The difference in trends after diagnosis between patient groups was not statistically significant, however (−0.0004; 95% CI, −0.002 to 0.001, P = .60). These findings were robust to the use of age as a continuous variable in the propensity score–matching process.
Trends in cumulative morbidity before and after diagnosis among patients with COVID-19 and historical control patients with ILI overall.
ILI = influenza-like illness.
Note: Based on 27,960 patients with COVID-19 (from 2020-2021) and 27,960 historical control patients with ILI (from 2018). Diagnosis was date of first primary care visit with the diagnosis code.
In the secondary analysis using the restricted cohort of ILI control patients, cumulative morbidity for both groups was similar at the time of diagnosis, whereas the divergence during the 6 months after diagnosis was greater than that in the primary analysis (Figure 3). The mean number of conditions increased from 0.48 at diagnosis to 0.68 at the 6-month follow-up among patients with COVID-19 and from 0.46 to 0.59 among ILI control patients. After diagnosis, COVID-19 patients experienced greater monthly increases in cumulative morbidity (trend = 0.030; 95% CI, 0.028-0.031; P <.001) as compared with ILI patients (trend = 0.020; 95% CI, 0.019-0.021; P <.001). Relative to their respective prediagnosis periods, both COVID-19 and ILI patients had different cumulative morbidity trends after diagnosis; the COVID-19 group had 0.006 fewer (95% CI, −0.008 to −0.004; P <.001) new diagnoses per month in the postdiagnosis period as compared with the prediagnosis period, while the ILI group had 0.015 fewer (95% CI, −0.016 to −0.014; P <.001).
Trends in cumulative morbidity before and after diagnosis among patients with COVID-19 and historical control patients with ILI who had a visit 180 days or more after diagnosis.
ILI = influenza-like illness.
Note: Based on 14,805 patients with COVID-19 (from 2020-2021) and 14,805 historical control patients with ILI (from 2018). Diagnosis was date of first primary care visit with the diagnosis code.
Comparison With Contemporaneous Controls
We identified 13,259 patients with COVID-19 in 2020 and 14,948 in 2021 who met the criteria for study inclusion. There were 754,846 patients in 2020 and 609,496 patients in 2021 who met inclusion and exclusion criteria to serve as contemporaneous controls having a wellness or preventive visit (Table 2). After matching, we retained 13,248 patients with COVID-19 and 13,428 wellness control patients in 2020, and 14,925 of each in 2021.
Prematched Characteristics of Patients With COVID-19 and Contemporaneous Control Patients With a Wellness or Preventive Visit
The COVID-19 patients and wellness control patients had modest differences in prevalence of the various diagnoses (Figure 1B). In 2020, patients with COVID-19 had greater morbidity with respect to breathing difficulties (2.5% vs 1.1%) and type 2 diabetes (6.3% vs 5.2%) as compared with control peers. Findings were essentially the same in 2021: the COVID-19 group again had higher prevalences of breathing difficulties (2.0% vs 0.8%) and type 2 diabetes (5.1 vs 4.5%).
DISCUSSION
In a typical month, 8 to 12 times more people receive care in primary care clinics than in emergency departments or hospitals, and primary care is where more than one-third of all patient visits occur.20,21 Primary care data offers a unique opportunity to assess health care use for the treatment of PCC, particularly for mild to moderate problems requiring medical attention but not necessarily elevated care that would necessitate acute care intervention. Our findings suggest that in the primary care setting, PCC occurred slightly more often than similar conditions that occurred after ILI or wellness visits, although these differences were modest. PCC symptoms were captured for only 12% of patients after COVID-19 infection. Our findings also suggest that the prevalence of PCC in this health care setting is less than 12% for the 17 assessed diagnostic categories at 6 months of follow-up.
The rates we observed are in stark contrast to those in most of the literature on the prevalence of PCC. For instance, systematic reviews report a prevalence of shortness of breath exceeding 20% (36.0% as found by Nasserie et al14 and 39.5% as found by Malik et al22 and Lopez et al23). Among our patients with COVID-19, 4.4% had documentation of breathing difficulties in the electronic health record. Similarly, the prevalence of fatigue from systematic reviews has been 40% or higher (40% as found by Nasserie et al,14 and 64% [95% CI, 54%-73%] as found by Malik et al22).23 In contrast, our patients had a 5.0% prevalence of documented fatigue after COVID-19.
There are possible explanations for the lower prevalence of PCC in our study as compared with others. First, patients in the American Family Cohort may be healthier and have fewer medical comorbidities before a COVID-19 diagnosis than patients seen in specialty or acute care settings. Numerous preexisting comorbidities, including chronic obstructive pulmonary disease, anxiety and depression, diabetes, and migraine, have been found to be associated with an increased risk of PCC.10,24 Second, greater COVID-19 severity is a known risk factor for PCC15; our study patients presented to primary care rather than an acute care setting. A tendency to include mainly patients hospitalized for COVID-19 treatment is a known critique of existing literature on PCC.14,22 Third, our study captured conditions using only ICD-10-CM codes documented in patients’ electronic health records, which could underestimate prevalence compared with patient self-report or clinical assessments in prospective studies. This undercoding of conditions should be expected to similarly affect the 2 comparison cohorts (ILI and wellness cohorts). Future work should explain PCC across a broader array of patient settings so that we have a more exhaustive picture of its epidemiology.
Our study has limitations. We restricted the sample to patients seen in the year before diagnosis to observe underlying health conditions; however, excluded patients may have been healthier patients less likely to seek care or patients with barriers to accessing care. Additionally, there are limitations to propensity score matching, including the assumption that the matching covariates capture the observable confounding variables. Also, we were not able to assess the severity of the incident condition, evaluate the role of vaccination on PCC among patients with COVID-19, or account for changes in clinical care capacity within clinics that may have affected study findings.
Our findings using a national primary care registry are the first to underscore the importance of describing COVID-19 patients’ morbidity in the general US population and highlight the importance of continued monitoring of PCC in primary care settings to understand its epidemiology, morbidity, and duration.
Acknowledgments
The authors acknowledge guidance from Drs Hector Bonilla, Linda Geng, and Robert Shafer at the Stanford University Post-Acute COVID-19 Syndrome (PACS) Clinic. The authors acknowledge Ms Lusilda Agolli for her diligence with formatting and preparing the manuscript for submission to the journal. The authors at Stanford University and the American Board of Family Medicine acknowledge funding support from the Centers for Disease Control and Prevention.
Footnotes
Conflicts of interest: Mr Kamdar maintains an appointment at the University of Michigan-Ann Arbor and a consulting engagement with the University of North Carolina-Chapel Hill. All other authors report none.
Funding support: This work was supported through a grant from the Centers for Disease Control and Prevention awarded to the American Board of Family Medicine in response to Opportunity 00HCPNED-2021 to 55473. Contract #75D30121P10944.
Disclaimer: The findings and conclusions in this report are those of the authors.
- Received for publication May 5, 2023.
- Revision received January 16, 2024.
- Accepted for publication March 4, 2024.
- © 2024 Annals of Family Medicine, Inc.