Abstract
PURPOSE The purpose of this study was to evaluate the effectiveness of collaborative trauma-informed care (CTIC) for treating depression in primary care in Chile.
METHODS From August 2021 through June 2023, 16 primary care teams in the Maule Region of Chile, were randomly assigned to either the CTIC or usual treatment (UT) group. At baseline, 3 months, and 6 months, 115 patients in the CTIC group, and 99 in the UT group, were blindly evaluated. The primary outcome was reduction in depressive symptoms. Secondary outcomes included improvement in anxiety symptoms, interpersonal and social functioning, emotional regulation, and adherence. Intention-to-treat data analysis, using analysis of covariance was conducted.
RESULTS There were 214 patients recruited; 85% were women, and 61% had 4 or more adverse childhood experiences. At 6 months, depressive symptoms declined significantly in the CTIC arm relative to UT (adjusted mean difference [AMD]= −3.09, 95% CI, −4.94 to −1.23; d = −0.46, 95% CI,−0.73 to −0.18; P = .001). Anxiety symptoms exhibited a trend toward improvement in the CTIC vs UT group (AMD = −1.50, 95% CI, −3.03 to 0.31; P = .055). No significant differences were observed in other secondary outcomes, except for adherence, which was significantly higher in the CTIC vs UT groups (AMD = 2.59, 95% CI, 1.80-4.99; P = .035).
CONCLUSIONS The CTIC approach demonstrated superior outcomes in treating depression and improving adherence compared with UT. Moreover, the observed trends in anxiety improvement warrant further exploration in future research with a larger sample size. It is necessary to assess the effectiveness of this approach in treating more complex, difficult-to-treat forms of depression.
- adverse childhood experiences
- collaborative care
- depression
- primary health care
- randomized control trial
BACKGROUND
In Chile, and globally, depression is a substantially disabling public health concern, disproportionately affecting women at twice the rate of men.1,2 Before the COVID-19 pandemic, studies indicated that 18.2% of Chilean adults experienced depressive symptoms, with 6.2% meeting the criteria for major depression.3-4 During the pandemic, depressive symptoms surged by 40.2%.5
Since 2006, Chile’s national mental health program has focused on depression, with primary care managing 90% of cases and referring individuals with actual suicidal conduct, suspected bipolar disorder, or signs of psychosis to specialists.6.7 Remission rates at primary care level are about 55%.8 Research indicates that one-half of the primary care patients exhibit a complex form of depression, characterized by comorbidities, suicide attempts, interpersonal difficulties, impaired social functioning, and adverse childhood experiences (ACEs), leading to worse outcomes.9,10 These patients receive minimal interventions, averaging just 2 medical and psychological sessions per year.10 This evidence highlights a critical gap in research and practice in Chilean primary care regarding the management of depression and its more complex presentations.11,12
The complex form of depression in Chilean primary care9-12 has characteristics similar to treatment-resistant depression (TRD), defined as an inadequate response to at least 2 antidepressants despite adequacy of the treatment trial and adherence to treatment.13 Treatment recommendations for TRD primarily involve pharmacotherapy.13,14 Experts recommend redefining TRD as difficult-to-treat depression (DTD) that requires a chronic care approach focused on symptom management and functional recovery.15,16 Trauma-informed care principles are also proposed because of the impact of trauma and socioeconomic factors on depression and suicidal tendencies.17-19
Both DTD and trauma-informed care approaches, support a collaborative care model for chronic diseases, which involves case managers, structured patient-centered approaches with scheduled follow-ups, and interprofessional communication.20,21 Implementing a training program for multidisciplinary teams, integrating a bio-psychosocial approach with trauma-informed care and collaborative care, is proposed to improve depression outcomes in primary care in Chile.
Our aim was to compare the effectiveness of collaborative trauma-informed care (CTIC) for depression vs usual treatment (UT) on clinical and functional outcomes in a cluster randomized clinical trial (RCT) deployed in primary care clinics in the Maule Region of Chile.
METHODS
Study Design
A single blind, 2-arm RCT was conducted from August 2021 through June 2023 in 16 primary care centers in the Maule Region of Chile. The trial protocol was approved by the Institutional Review Board of the University of Talca and registered in the clinical trial registration NCT05016388 (https://clinicaltrials.gov). The study (SAI200031) was funded by the Chilean National Research and Development Agency Health Research and Development Fund (ANID-FONIS).
The 16 primary care centers were matched by socioeconomic status and patient income and blindly randomized by 1 of the investigators (M.L.A.) using a pseudorandom algorithm in MATLAB (MathWorks, Inc). The centers were blindly assigned to the CTIC or UT group (Figure 1, Supplemental Appendix).
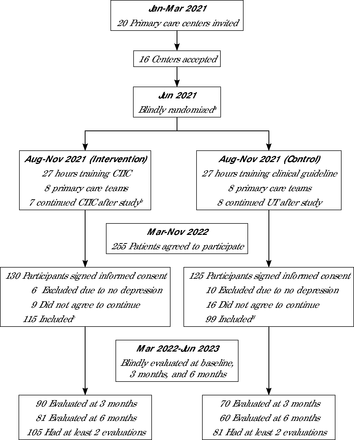
Flowchart of recruitment process.
CTIC = collaborative trauma-informed care; UT = usual treatment.
aThe 16 centers were coded with letters A-P, then randomly assigned to the intervention (A, C, G, H, I, K, M, O) and control groups (B, D, E, F, J, L, N, P).
bThe center coded C did not continue to use CTIC procedures after the study.
cNumber of intervention participants by center code was A 13, C 0, G 23, H 18, I 15, K 13, M 10, O 23.
dNumber of control participants by center code was B 14, D 26, E 19, F 9, J 14, L 8, N 9, P 3.
Study Groups
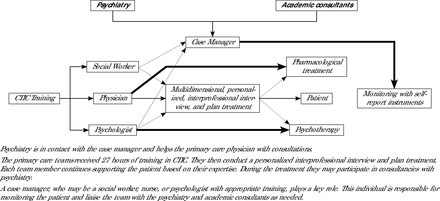
The CTIC teams comprised of a physician, psychologist, and social worker received 27 hours of training on the CTIC approach (intervention), emphasizing trauma-informed care and DTD,19,22,23,24 while respecting current clinical guidelines. (Table 1, Supplemental Appendix). The training was conducted by V.G.V., A.C., and A.F.S. After training, teams applied the model with a case manager, personalized patient interviews, validated self-report instruments, and monthly psychiatrist supervision for 3 months (Figure 2, Supplemental Appendix).
CTIC Training for the Treatment of Depression in Primary Care
Collaborative trauma-informed care for treating depression in primary care.
CTIC = collaborative trauma-informed care.
The UT group primary care professionals received 27 hours of training on UT (control), conducted by Jorge Calvo, MD and Antonio Arellano, MD on the current Depression Treatment Guidelines6 (Supplemental Appendix).
Participants
Considering previous studies, a difference of 20% between arms, an α level of 5%, a power of 80%, a confidence level of 95%, and a maximum variance of 50%, an initial sample of 394 patients from 8 clinics was calculated10,25,26 (Supplemental Appendix). Due to the COVID-19 pandemic, however, the number of centers was adjusted to 16.
Adults aged 18 to 70 years admitted for treatment of depression in primary care clinics of the Maule Region with a confirmed diagnosis of depression, according to the Mini-International Neuropsychiatric Interview (MINI),27 were included in the study. Those with sensory disabilities, no access to a telephone, who had been referred to a specialist level, or were unable or unwilling to sign the informed consent, were excluded from the study.
Procedures
At each clinic, a member of the primary care team recruited study participants who provided written informed consent. These participants were then referred to 2 psychologists (Marcela Ormazábal, BS, S.B.) who were kept unaware of the patients’ assigned group and allocated them to an external evaluation team made up of 10 psychiatry residents. A protocol for managing emergency cases was established (Supplemental Appendix).
The residents received a 4-hour training session on data standardization and were supervised by Marcela Ormazábal, BS and S.B. The participants were evaluated at the time of inclusion using a semi-structured clinical interview, the ACEs inventory,28 the MINI,27 and a set of instruments to assess outcomes at baseline, 3 months, and 6 months.
Primary Outcome
Depressive symptoms were assessed using the 9-item Patient Health Questionnaire (PHQ-9), validated in Chile, with scores ranging from 0 to 27. A score of 7 or higher indicates the presence of depressive symptoms.9,29,30
Secondary Outcomes
Anxiety symptoms were evaluated using the Spanish version of the 7-item Generalized Anxiety Disorder scale (GAD-7). Scores range from 0 to 21. Scores of 10 points or more indicate anxiety symptoms.31
Emotional regulation was evaluated through the Spanish validated version of the Difficulties in Emotion Regulation Scale (DERS), with scores ranging from 0 to 140 points. Scores of 73 points or more indicate emotional deregulation symptoms.32
Interpersonal and social functioning was evaluated with the interpersonal and social role subscales included in the validated Outcome Questionnaire 45 (OQ45). The interpersonal subscale ranges from 0 to 48, with scores of 16 points or more indicating interpersonal dysfunction. The social subscale ranges from 0 to 36 points, with scores of 14 points or more indicating social role dysfunction.33
Therapeutic adherence was measured at 3 and 6 months using a brief version of the General Health Adherence Scale (GHAS), consisting of 12 items with scores ranging from 0 to 36. This self-report scale assesses general attitudes and behaviors toward treatment as a whole. Scores below 24 indicate low adherence, scores between 25 and 30 indicate moderate adherence, and scores above 31 indicate high adherence. This scale was previously validated in Chile.34
The data collected from the instruments at baseline, 3 months, and 6 months was entered into a secure virtual worksheet hosted on the website https://www.surveymonkey.com/ (Momentive Global).
Statistical Analysis
We performed intention-to-treat analyses for all clinical outcomes using the Consolidated Standards of Reporting Trials (CONSORT) guidelines for RCTs.35
We analyzed the primary and secondary outcomes after the 6 month follow-up using an analysis of covariance (ANCOVA) controlling for the individual baseline values for each outcome. Two assumptions of the ANCOVA were tested; the absence of differences between groups in the baseline data, and the homogeneity of regression for baseline and 6 months follow-up data. Before running the ANCOVA, we evaluated the intraclass correlation coefficient (ICC) for each outcome, at baseline and 6 months, except for adherence, which was evaluated at 3 and 6 months follow-up. The ICC was estimated as the between subject variance divided by the total (between plus within subject) variance from the analysis of variance (ANOVA). The effect size we calculated using the Cohen’s d measure and its 95% CI.
The entire statistical analysis was conducted using SPSS version 21 (IBM Corp) and jamovi version 2.5 (The jamovi project 2024) following a predefined analysis plan. For sensitivity analysis, missing data were addressed through imputation based on the Last Observation Carried Forward method. There were no significant baseline differences in missing data between the 2 groups or between those retained.
RESULTS
Participant Flow and Retention
We invited 20 primary health care centers, of which 16 participated; 8 were trained in CTIC and 8 in UT. Seven centers continued their operation according to CTIC protocol (Figure 2). The CTIC groups reported a mean of 16.4 participants and a variance of 23.6, compared with UT centers with a mean of 14.1 participants and a variance of 42.9.
We recruited 214 patients; 115 in the CTIC group, and 99 in the UT group. At the 3-month follow-up, data were collected from 81% of the CTIC group and 70% of the UT group (Z = 1.26, P = .87). At the 6-month follow-up, data were collected from 70% of the CTIC group and 61% of the UT group (Z = 1.17, P = .20; Figure 1). At least 87% of the sample had more than 1 evaluation (91% in the CTIC group and 82% in the UT group, Z = 8.83, P <.01).
Five emergency situations were detected and satisfactorily resolved.
Baseline Characteristics of Participants
The majority of the participants were women (182 of 214; 85%), with a mean age of 40.1 years (SD 13.5) for the CTIC group and 40.2 years (SD 15.8) for UT group. Table 2 shows sociodemographic and clinical characteristics. Both groups were similar in all aspects, demonstrating homogeneity between the groups at baseline.
Baseline Clinical and Sociodemographic Characteristics of Participants (N = 214)
Primary Outcome
We observed a significant decrease in PHQ-9 scores over time; in both the CTIC and UT groups, (η2 = 0.3, P <.001). The CTIC group’s scores decreased from a mean (SD) of 17.1 (5.7) at baseline to 8.9 (6.6) at 6 months, while the UT group’s scores dropped from 17.3 (6.1) to 12.2 (8.1) (Table 3). A significant difference in depressive symptoms between the groups over time was found (η2 = 0.049, P <.01; Table 3). The CTIC group showed an improvement at 6 months (adjusted mean difference [AMD] = −3.09, 95% CI, −4.94 to −1.23; d = −0.46, 95% CI, −0.73 to −0.18; P = .001), with an ICC at baseline of 0.06 that increased to 0.10 at 6 months follow-up, in agreement with a decrease in the depressive symptoms (Table 4).
Longitudinal Assessment Intervention vs Control Groups at Baseline, 3 Months, and 6 Months
Comparative ITT Analysis of Intervention vs Control Groups: Impact on Outcomes and Adherence at 6 Months
At 6 months, 62 of 115 patients (54%) in the CTIC group showed remission, compared with 34 of 99 patients (34%) in the UT group (OR = 2.24; 95% CI, 1.58-3.20).
Secondary Outcome
Both groups showed improvement in all measured variables: anxiety symptoms (η2 = 0.14, P <.001), interpersonal functioning (η2 = 0.07, P <.001), social role (η2 = 0.05, P <.001), and emotional regulation (η2 = 0.17, P <.001). No significant differences between time and group were observed in these outcomes (Table 3), and the intracluster analysis indicated no differences between centers (Table 4). The statistical analysis showed that the effects of the intervention did not extend to the secondary outcomes (Table 4).
Interestingly, the adherence scale to treatment in participants from the CTIC group was 2.5 points higher (95% CI, 1.8 to 4.99; P = .035) at 6 months. In this scale, the ICC = 0.10 at 3 months and increased to 0.11 at 6 months, suggesting differences between the different centers included in the groups.
DISCUSSION
This clinical trial is an innovative effort that demonstrates the effectiveness of an educational program for improving depression outcomes, by integrating a multidimensional approach with a trauma-informed care perspective. Despite enrolling only 214 participants (54% of the planned sample size of 394) due to difficulty recruiting patients during the COVID-19 pandemic, the effect size (d = −0.46) for depressive symptom improvement of was consistent with comparable studies.36-39
The study population, mostly women with moderate to severe depressive symptoms, high anxiety comorbidity, and elevated ACEs rates, aligns with pre-pandemic Chilean studies.10, 40-45 In a study, conducted in the same disadvantaged region,45 remission rates were 43% at 6 months and 52% at 1 year.10 With the onset of the pandemic, primary care shifted its focus toward emerging health issues, overshadowing adult mental health,46 which contributed to challenges in recruitment of adult patients with depression, increased variability of inpatient admissions (particularly in the UT group), and contributed to only a 34% remission rate in the UT group. In contrast, CTIC teams achieved a 54% remission rate at 6 months, matching the 12-month rate observed in an earlier study.10 This 20% difference highlights the potential efficacy of the CTIC approach in the post-emergent COVID-19 pandemic period. Likely, primary care teams in the UT group lacked the comprehensive assessment tools and psychosocial recommendations emphasized by the intervention team, enabling those within the CTIC framework to more effectively manage adult depression under the circumstances.47,48
The improvement in the general adherence scale within the CTIC group compared with the UT group at 6 months is significant, albeit with a small size effect. This finding warrants further investigation into the specific factors within each primary care team that influenced their success or shortcomings. Additionally, both groups demonstrated high follow-up rates, exceeding 80%, underscoring the feasibility of our study and the reliability of the data collected. Notably, the CTIC group exhibited greater adherence to blind follow-ups, likely attributed to the more effective patient management and engagement strategies of the CTIC approach.
Our intervention, based on trauma-informed care,19,22-24 highlights the relevance of including a sensitive inquiry about any adverse biographical history in the patient’s multidimensional evaluation, and in the structure of the treatment and follow-up sessions that establish a safe patient-care team relationship.19,22-24 This approach aligns with what is proposed by authors who emphasize the critical role of ACEs in diagnosis, treatment, and education.49-51 Moreover, it underscores the importance of integrating psychotherapy with pharmacotherapy to treat DTD, consistent with several studies.52-54 These crucial aspects have not been fully integrated into current practices or treatment recommendations, as evidenced in a Latin American consensus guideline that predominantly emphasizes biomedical approaches, and excludes psychotherapy from their treatment protocol.14
Regarding the secondary outcomes, we noticed a trend toward improvement in anxiety; however, statistical significance was not reached. Existing evidence suggests that the resolution of anxiety in depression in primary care typically occurs later in the course of treatment.55,56 Furthermore, the gradual decrease in both depressive and anxiety symptoms, especially within the CTIC group over time, may be linked to a greater integration of the model by the teams, potentially yielding improved results with a longer follow-up time.
Moreover, there were no significant differences between groups in interpersonal difficulties and emotional regulation. The CTIC treatment takes a generalized training approach, focusing on non-specific competencies within primary care teams for managing mental health disorders. To effectively address interpersonal and emotional deregulation in patients with complex depression, primary care professionals may need to acquire specific skills, as emphasized in a recent training program for psychologists.57
This study had several limitations, including a gender imbalance with a majority of female participants, and the potential negative impact of the ongoing COVID-19 pandemic on depression treatment in the UT arm. In addition, specific interventions in both study groups, such as number of physicians in each team, type and frequency of psychological interventions, and use of psychotropic medication, were not accounted for. A second analysis is necessary to evaluate the specificity of this intervention in patients exhibiting the characteristics of complex DTD. Thus, additional studies should address the feasibility and acceptability of CTIC implementation in different contexts.
In summary, this pioneering study represents a groundbreaking initiative for managing depression in primary care in Chile. The CTIC treatment addresses often neglected aspects with a flexible design, allowing various professionals to acquire essential skills while emphasizing interprofessional collaboration. Moving forward, it is essential to continue generating evidence to inform decision-making processes and the incorporation of skills aimed at recognizing and managing prevalent variables in depression, which are typically excluded from clinical practice. The comprehensive approach of CTIC should contribute to ongoing improvements in mental health services within primary care settings.
Acknowledgments
We would like to express our gratitude to Dr Jorge Calvo, Antonio Arellano, Sergio Guiñez-Molinos, and Johanna Kreither for their invaluable collaboration in the capacitation process. We also extend our appreciation to Marcela Ormazábal for her assistance in the recruitment process of patients. Our sincere thanks go out to the Health Secretaries of the Municipalities of Curicó, Constitución, Linares, Pelarco, Romeral, Sagrada Familia, and Talca, as well as all the mental health teams and directors of the primary care clinics who played a crucial role in this project. Special thanks also to Carmen Gloria Blanco for her exceptional project management assistance.
Footnotes
Conflicts of interest: authors report none.
Funding support: The study was funded by the agency ANID - FONIS (Project SAI200031)
Author contributions: V.G.V., A.C., A.F.S. conceived the study; V.G.V., A.C., S.B., M.L.A. participated in project management; M.L.A. analyzed the data; all authors contributed to the writing of the manuscript and approved the final version.
- Received for publication February 2, 2024.
- Revision received August 5, 2024.
- Accepted for publication August 14, 2024.
- © 2024 Annals of Family Medicine, Inc.