Abstract
PURPOSE Although potentially costly, enhancing primary care depression management on an ongoing basis results in substantial long-term treatment effectiveness. The purpose of this article is to compare the cost-effectiveness of this approach with that of usual care.
METHODS The study was conducted in 12 community primary care practices randomized to enhanced or usual care after stratification by baseline practice patterns. Practices assigned to enhanced care encouraged depressed patients to engage in active treatment, using practice nurses to provide regularly scheduled care management during the course of 24 months. We analyze outcomes for 211 adults (73.4% of potential eligible patients) beginning a new treatment episode for major depression determined by previsit screening. Outcomes included blinded estimates of days free of depression impairment as well as health care costs for 2 years.
RESULTS Enhanced care significantly increased the number of days free of depression impairment for 2 years when compared with usual care (647.6 days vs 588.2 days, P <.01). The incremental cost-effectiveness ratio for enhanced care ranged from $9,592 to $14,306 per quality-adjusted life-year (QALY). The number of incremental days free of depression impairment increased between the first year and the second year (23.0 vs 36.4, respectively, P <.001) while incremental health plan costs decreased significantly ($568 vs -$12, P <.001).
CONCLUSIONS Enhancing primary care depression management on an ongoing basis should be considered for adoption by policy and health plan leaders.
INTRODUCTION
Depression is a leading cause of disability in developed nations.1 Most primary care depression programs designed to improve acute depression management last 6 months or less.2–,22 Because brief programs have little to no sustained effect 1 year after termination,21–,24 we tested a model that enhances primary care depression management on an ongoing basis. By supplementing acute management25 with systematic monitoring for 24 months, this model incorporates chronic disease management principles26,27 and results in clinically significant improvements in both symptoms and functioning at 2 years.28 Given the clinical effectiveness at modest cost, we hypothesized that enhancing primary care depression management on an ongoing basis would be cost-effective when compared with usual care. Although it is premature to draw definitive conclusions when comparing the relative costs of brief and ongoing models of care, evidence of the cost-effectiveness of ongoing models provides important new information about the value of extending the brief programs currently being disseminated.29
Because the model we tested did not improve clinical outcomes in patients who entered the study depressed despite recent treatment,30 we reasoned that health care managers interested in this model would target it to patients who benefited, eg, patients beginning a new depression treatment episode. Thus, we conducted a post hoc analysis of randomized trial data drawn from patients beginning a new depression treatment episode.
METHODS
Experimental Design and Sample
Our methods, described in detail elsewhere,25 are summarized here. After approval by the Human Research Advisory Committee of the University of Arkansas for Medical Sciences and the Colorado Multi-Institutional Review Board, the research team conducted the study in 12 community primary care practices across the United States, none of which employed onsite mental health professionals to treat depression. The first author matched the 12 practices into 6 blocks by depression treatment patterns, and 1 practice from each block was randomly assigned to enhanced care. Patients coming in for routine visits at these practices between April 1996 and September 1997 were asked to complete a 2-stage screening instrument. Patients eligible for the study reported 5 or more of the 9 Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R31 criteria for major depression in the preceding 2 weeks. Excluded from the study were eligible patients who met criteria for bereavement, mania, alcohol dependence, pregnancy or postpartum, or life-threatening physical illness; patients who did not intend to use the clinic as their usual source of care in the next year; patients who did not have telephone access; patients who were illiterate in English; or patients who were cognitively impaired. For this analysis, we also excluded depressed patients in treatment at baseline.
Enhanced Care
Before patient enrollment, the research team provided brief training25 to primary care professionals in enhanced care practices to implement a care management system to improve acute and continuation phase treatment for patients with major depression. Training emphasized that physicians and care managers encourage patients to initiate guideline-concordant pharmacotherapy or psychotherapy through regularly scheduled contacts during the acute phase of treatment.30 Training also emphasized that physicians and care managers make regularly scheduled contacts to encourage continued treatment adherence when symptoms were resolving, to adjust treatment if symptoms were not resolving, and to terminate treatment when patients in remission did not require maintenance therapy after the continuation phase of care.28 Care managers reached 95.7% of 115 patients in enhanced care practices during the 24 months of the study, for an average of 11.8 contacts (SD = 5.9) per patient. Usual care practices were not systematically informed about which patients were participating in the study.
Data Collection
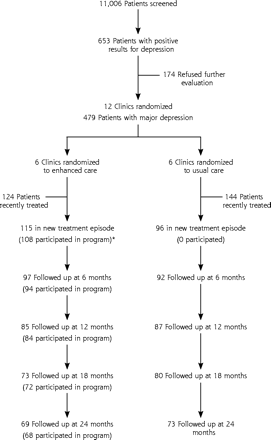
As displayed in Figure 1⇓, 653 of 11,006 patients had positive screening results for depression on the 2-stage screening instrument and met study criteria for enrollment. Of these patients, 73.4% (479 of 653) agreed to further evaluation, and 44.0% (211 of 479) were beginning a new treatment episode for major depression. After screening, all data were collected during a structured telephone interview by an independent research interviewer. The interviewer was unaware of whether the patient was receiving enhanced treatment or usual care, except for 3 patients whose practices were contacted for updated location information. Concordant with an intent-to-treat design, patients who left the practice were interviewed again even though they could not continue to participate in ongoing intervention. Follow-up interviews at 6, 12, 18, and 24 months conducted by the research interviewer between October 1996 and September 1999 achieved response rates of 89.6%, 81.5%, 72.5%, and 67.3%, respectively.
Patient recruitment and participation flowchart.
* A total of 110 patients received any program contact, including 2 patients who participated after 6 months only.
Operational Definition of Major Constructs
Quality-Adjusted Life-Years
We evaluated the impact of the program on days free of depression impairment by asking patients to estimate the number of days during the past 4 weeks that their emotional problems kept them in bed all or most of the day or caused them to cut down on activities they usually do for 1 half-day or more. We estimated the impact of the model on quality-adjusted life-years (QALYs) by assuming that each depression impairment-free day improved QALYs by .00082 (.3/365), consonant with estimates in the literature that patients realize a .2 to .4 decline in QALYs with 365 days of depression.32–,35 We also estimated the impact of the program on generic measures of QALYs. A description of the analysis is available online only as supplemental data in the Appendix,36–,42 which can be found at: http://www.annfammed.org/cgi/content/full/3/1/7/DC1.
Costs
We assumed an accounting perspective43 to estimate the cost of enhanced care to society and to health care plans. To describe its cost to society in the health care environment in which it was tested (nonadjusted cost), we estimated the difference between enhanced and usual care in actual program costs, outpatient costs, and patient time and transportation costs for 2 years; for its cost to health care plans, we estimated the difference between enhanced and usual care in actual program and outpatient treatment costs for 2 years. To describe its effect in the 2007 health care environment, when commonly prescribed antidepressant medications are expected to cost considerably less than they do today (medication-adjusted costs), we estimated outpatient treatment costs using generically priced selective serotonin reuptake inhibitors.
Cost data were log-transformed to approximate normal distributions before analysis, and retransformed using appropriate smearing retransformations.44,45 All costs reported in this article (for our study and other studies) have been adjusted by the Consumer Price Index adjustment46 to reflect 2000 US dollars.
Program costs included time costs (salary plus fringe benefits) derived from care manager logs for patient screening, preparation for and delivery of enhanced care, record keeping and review, and care manager-physician communication plus overhead. Our annual per capita model cost estimates, summarized in Table 1⇓, differ slightly from our estimates in Table 2⇓, reflecting the uncertainty introduced in the transformation-retransformation process of modeling costs.
Derivation of per Capita Program Costs (Nonadjusted)
Enhanced Care Impact on Incremental Costs by Category and Year (n = 211)
Outpatient Costs.
Similar to previous studies,32 outpatient costs for primary care and specialty mental health care visits, emergency department visits, and psychotropic medication were estimated from patient-reported utilization at each wave, reflecting that patients were insured by 65 different health plans or uninsured. Outpatient visit costs were estimated using 1999 Medicare payment rates. Emergency department visit costs were estimated at $500 per episode. For the nonadjusted cost estimate, psychotropic medication costs were priced at the lowest average generic wholesale price per medication dosage reported in the 2000 Red Book47 of prescription drugs. For the medication-adjusted cost estimate, fluoxetine, sertraline, and paroxetine were priced at $0.50 per dose. Like other cost-effectiveness analyses of primary care depression models,2,4,10 we did not include (1) inpatient costs, because we did not observe between-group differences in hospitalization, which affected only a small proportion of patients; and (2) productivity costs, often attributed to the nonmonetized denominator in the cost-effectiveness ratio,37 the exclusion of which results in a conservative estimate of the cost-effectiveness of the model from a societal perspective.
Patient Time and Transportation Costs.
Time costs were estimated from patient reports of travel times to and from the clinic plus waiting time. For employed patients, time costs were calculated using self-reported wage rates. For unemployed patients, we substituted year 2000 average wage rates by gender and education as a proxy of patient time costs. Transportation costs were calculated from patient-reported round-trip miles to and from the location of services at a rate of $0.325 per mile.
Cost-Effectiveness Ratio.
The numerator in the cost-effectiveness ratio is the incremental difference in cost between enhanced and usual care. The denominator is the incremental difference in QALYs between enhanced and usual care. Costs and QALYs were not discounted, because costs and benefits accrue concurrently during the short time horizon of the study.
Data Analysis
We conducted intent-to-treat analyses controlling for clinical and sociodemographic covariates using all available data to evaluate the incremental effect (enhanced minus usual care) of enhanced care on depression impairment-free days and cost using the perspective of a typical patient in our sample (see the Appendix). When these models indicated enhanced care had a significant impact on both outcomes and cost, we used a bootstrap method48,49 across 1,000 replications (1) to generate the distribution of QALY, costs, and cost-effectiveness ratios with nonparametric confidence intervals; and (2) to construct dominance plots and acceptability curves50 to describe the distribution of enhanced care cost-effectiveness in the current health care environment.
RESULTS
Study Participants
On average, the 211 patients participating in the study were 43.1 years old (SD = 14.8 years), with 84.4% female, 15.6% minority, 47.4% currently married, 79.2% high school educated, 62.1% employed full-time or part-time, and 82.5% with health insurance. These patients had an average of 2.1 physical comorbidities. Clinically, they reported an average of 6.4 DSM-III-R depression criteria in the preceding 2 weeks, 10.0% met criteria for dysthymia in the past year, and 73.3% reported a previous episode of depression. Enhanced and usual care patients were comparable except for small but statistically significant differences in age, depression severity, and physical comorbidity, which were controlled for by covariates in the model (described in the Appendix).
Enhanced Care Effects on QALYs
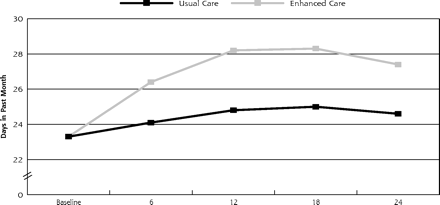
As displayed in Figure 2⇓, there were significantly more days free of depression impairment during the 2 years for enhanced care patients than for usual care patients (647.6 days vs 588.2 days, P <.01). In the bootstrap sample, enhanced care increased incremental days free of depression impairment during the 2 years by 59.4 days (95% confidence interval [CI], 38.0–80.7), with enhanced care patients reporting significantly more incremental days free of depression impairment in the second year than in the first (36.4 days and 23.0 days, respectively, P <.001). Translating these effects to quality of life, the impact of enhanced care on depression-specific QALYs relative to usual care for the 2 years was 0.049.
Enhanced care effect on outcomes (n=211).
Enhanced Care Effects on Costs
Table 2⇑ displays the impact of enhanced care on costs by category and year. Total nonadjusted costs (program, outpatient, and patient time and transportation costs) increased by $701 more than usual care (95% CI, $637-$765, F = 6.82, P >.001, df = 4,198). The effect of enhanced care on total nonadjusted costs was significantly greater in the first year than in the second ($675 vs $26, respectively, P <.001). Health plan nonadjusted costs (program and outpatient costs) increased by $556 more than usual care (95% CI, $500-$612, F = 7.46, P <.001, df = 4,198). The effect of enhanced care on health plan nonadjusted costs was significantly greater in the first year than in the second ($568 vs -$12, respectively, P <.001), indicating reduced health care utilization from enhanced care was sufficient to pay for its cost the second year. Total medication-adjusted costs increased by $470 more than usual care (95% CI, $412-$527, F = 6.23, P <.001, df = 4,198), and health plan medication-adjusted costs increased by $325 more than usual care (95% CI, $275-$374, F = 6.86, P <.001, df = 4,198).
Cost-Effectiveness Ratios
The bootstrap analysis indicated that the mean incremental cost-effectiveness ratio for enhanced care ranges from $9,592 (medication-adjusted costs) to $14,306 (nonadjusted costs) per QALY. Dominance plot analysis showed that 88.7% of patients would be expected to have improved outcomes with increased costs, and 11.3% of patients would have improved outcomes with decreased costs. Acceptability curve analysis showed that the mean incremental cost-effectiveness ratio had a 100% probability of being less than $20,000 per QALY in all analyses.
DISCUSSION
Consistent with its sustained effectiveness,28 the cost-effectiveness ratio for improving primary care depression management for 2 years in this population ranges from $9,592 to $14,306 per QALY, slightly less than our first-year estimate.51 This analysis shows that incremental QALYs significantly increase with time while incremental costs decline, providing the first evidence that depression disease management can become more effective and less costly with time. We suspect we observed increasing QALYs because enhanced care improved role functioning until it reached nearly normal levels at 2 years.28 Improved functioning may have, in turn, caused or been simultaneous with a reduction in outpatient costs sufficient to cover modest program costs, as we observed in the second year.
Although direct comparisons cannot be made with previous primary care depression trials that use varying strategies to measure cost-effectiveness in clinically different populations (Table 3⇓2,4,10,33,34), enhanced care management on an ongoing basis appears to be a particularly promising use of health care resources. This conclusion is compatible with published observations that the clinical improvement associated with brief programs declines with time,24,35 whereas the clinical improvement associated with ongoing management increases with time.28
Cost-Effectiveness of Primary Care Programs for Depressed Patients Beginning a New Treatment Episode Compared with Usual Care
This debate over brief vs ongoing management models should not obscure the recognition that cost-effectiveness ratios associated with both models suggest improving primary care depression management is a good value and results in comparable or greater cost-effectiveness than smoking cessation counseling (>$8,000 per QALY), hypertension pharmacotherapy (>$14,000 per QALY), hypercholesterolemia pharmacotherapy (>$18,000 per QALY), chronic obstructive pulmonary disease rehabilitation (>$36,000 per QALY), or depression screening alone (>$45,000).52–,54
While encouraging cost-effectiveness ratios are useful in convincing policy makers that providing high-quality primary care depression treatment is an efficient use of health care resources, they may be less helpful in elucidating which stakeholder group (purchasers, plans, or patients) should underwrite program and incremental treatment costs. These data suggest if purchasers did not contribute any additional monies for enhanced depression management, plans with access to multiple generically priced antidepressants would spend $383 ($158 + $225) the first year and save $58 ($130 – $188) the second year by providing ongoing management to each depressed participant. Plans may be able to pass a portion of these costs on to their patients, as depressed patients report being willing to pay an average of $1,620 for a 6-month treatment that eliminates all symptoms of depression.55
The internal validity of our findings is strengthened by a randomized block design to evaluate the impact of enhanced care on the outcomes and costs of care using an intent-to-treat analysis. Our conclusions about cost-effectiveness are strengthened by the convergence of estimates derived from depression-specific and generic QALY measures (see the Appendix). To address recently released recommendations to evaluate the impact of the intervention on functional status,56 we selected a depression-specific QALY measure that captured depressive symptoms severe enough to reduce functioning, recognizing that patients in both the enhanced and usual care condition may have additional days of milder symptoms than our measure captured. Because the 3 measures of depression-free days currently in use (Table 3⇑) conceptually and statistically overlap to varying degrees, we encourage future researchers to reach consensus on a brief and valid measure of depression-specific QALYs. Doing so will increase opportunities to compare program results across large effectiveness trials. Our cost estimates for the as-planned program25 are slightly higher than our actual cost estimates for the as-delivered program, because enhanced care patients did not participate in all the contacts offered. Fielding the study in practices caring for patients insured through multiple health plans forced us to rely on patient reports to measure outpatient costs. Relying on patient-reported health care use introduces measurement imprecision; however, it should not bias the study findings on incremental costs. Regarding our exclusion of nonpsychotropic medication (which is not available in the data set), we note that similar efforts to improve depression treatment have not had a significant impact on these costs,57 so we do not anticipate that excluding them from cost-effectiveness estimates seriously biased our results. Regarding our decision to exclude hospitalization costs, we encourage researchers to derive cost-effectiveness ratios including hospitalization costs in preplanned meta-analysis of multiple large trials, because hospitalizations are too infrequent an event in any single trial to estimate their contribution to incremental costs with any precision.
The generalizability of our findings is strengthened because the program was implemented by health care professionals practicing in organizationally heterogeneous clinics across the country under naturalistic conditions where physicians and diverse patients were free to select the treatments they preferred. We attempted to reduce the impact of sample loss so it was similar to or smaller than that of most studies of this kind24 by using modeling techniques that allowed us to project trends when patients did not complete all follow-up interviews. We emphasize that our results are limited to primary care patients beginning a new treatment episode for depression (about 44% of depressed patients visiting a practice), because our previous research shows that this approach is not effective for depressed patients receiving depression care at baseline.30 We encourage further research that tests approaches which increase specialty care consultation or collaboration2,58 for this potentially treatment-resistant cohort.
Acknowledgments
The authors wish to acknowledge the physicians, office staff, and patients of participating primary care practices: Chatham Primary Care, Siler City, NC; Dunes Family Health Care, Reedsport, Ore; Eau Claire Family Medicine, Eau Claire, Wisc; Enid Family Medicine Clinic, Enid, Okla; Fergus Falls Medical Group, Fergus Falls, Minn; Health East Eastside Medical Center, St. Paul, Minn; Lynchburg Family Practice, Lynchburg, Va; Mile Bluff Clinic, Mauston, Wisc; Mountain Area Family Health, Asheville, NC; Northern Colorado Family Medicine, Greeley, Colo; Oakwood Health Care Center, Westland, Mich; Somerset Family Practice, Somerville, NJ; and University of North Dakota Center for Family Medicine, Minot, ND. We are extremely grateful to our colleagues in the Depression Guidelines Study, the Quality in Depression (QID) Cooperative Agreement, and research staff from the Center for Outcomes Research and Evaluation for the superb technical assistance and encouragement they offered.
Footnotes
-
Conflicts of interest: none reported
-
Funding support: This project was supported by the National Institute of Mental Health MH54444 and MH63651, and the MacArthur Foundation. Dr. Pyne’s contributions were supported by a VA Research Career Development Award and the VISN 16 MIRECC.
- Received for publication March 19, 2004.
- Revision received June 1, 2004.
- Accepted for publication June 7, 2004.
- © 2005 Annals of Family Medicine, Inc.