Abstract
PURPOSE The purpose of this study was to evaluate differences in the management of cardiovascular disease (CVD) risk factors based upon the sex of the patient and physician and their interaction in primary care practice.
METHODS We evaluated CVD risk factor management in 4,195 patients cared for by 39 male and 16 female primary care physicians in 30 practices in southeastern New England.
RESULTS Many of the sex-based differences in CVD risk factor management on crude analysis are lost once adjusted for confounding factors found at the level of the patient, physician, and practice. In multilevel adjusted analyses, styles of CVD risk factor management differed by the sex of the physician, with more female physicians documenting diet and weight loss counseling for hypertension (odds ratio [OR] = 2.22; 95% confidence interval [CI], 1.12–4.40) and obesity (OR = 2.14; 95% CI, 1.30–3.51) and more physical activity counseling for obesity (OR = 2.03; 95% CI, 1.30–3.18) and diabetes (OR = 6.55; 95% CI, 2.01–21.33). Diabetes management differed by the sex of the patient, with fewer women receiving glucose-lowering medications (OR = 0.49; 95% CI, 0.25–0.94), angiotensin-converting enzyme inhibitor therapy (OR = 0.39; 95% CI, 0.22–0.72), and aspirin prophylaxis (OR = 0.30; 95% CI, 0.15–0.58).
CONCLUSION Quality of care as measured by patients meeting CVD risk factors treatment goals was similar regardless of the sex of the patient or physician. Selected differences were found in the style of CVD risk factor management by sex of physician and patient.
INTRODUCTION
Cardiovascular disease (CVD) remains the leading cause of morbidity and mortality in the United States for both men and women.1 The large number of epidemiological studies and clinical trials that have documented the benefits of treating dyslipidemia, diabetes mellitus, hypertension, and obesity using behavioral and pharmacological means have led to clinical practice guidelines aimed at implementing these management recommendations.2–8 Several investigators have suggested that there may be sex disparities in the management of CVD and its risk factors and in the implementation of CVD risk factor clinical guidelines in primary care practice.9–21 Differences in the management of hypertension, dyslipidemia, obesity, and diabetes mellitus based upon the sex of the patient16–21 and of the physician and by patient-physician concordance by sex22–25 have been reported. Findings indicate that male and female physicians differ in their communication skills, practice styles, time spent with patients during encounters, and frequency of providing preventive services.22–34 Few studies, if any, however, have examined the effects of the sex of the physician as a variable independent of other physician factors, such as years in practice, medical specialty, and the statistical effects associated with the clustering of patients by physicians in a practice in relation to CVD risk factor management.30–36 Because more than one-half of outpatient care in the United States occurs in the primary care setting,37 the purpose of this study was to conduct a multilevel analysis of the effects of the sex of the patient and physician, as well as physician-patient interactions by sex, on the management of CVD risk factors in primary care.
METHODS
Study Design and Data Collection
These analyses are based upon baseline data collected in 2004 as part of a cluster randomized trial aimed at testing the effectiveness of translating the Adult Treatment Panel III (ATP III)2 cholesterol treatment guidelines into primary care. The data collected were from 30 representative primary care physician practices in southeastern New England, comprising 55 primary care physicians and 4,195 patients. The data for these analyses came from medical record chart reviews, patient’s telephone interviews, and physician’s in-person questionnaires and interviews. The research and Health Insurance Probability and Accountability Act (HIPAA)38 protocol of physicians, staff, and patients were approved by the human subjects protection committee (Institutional Review Board) of Memorial Hospital of Rhode Island.
Practice and Patient Recruitment
To obtain a representative sample, letters were sent to all primary care physicians (n = 919) in southeastern New England. More than 100 physicians responded affirmatively, and 71 practices that were in close geographic proximity to the researchers were selected and stratified into large and small internal medicine and family medicine practices. From each of these strata, we randomly selected practices and obtained informed consent. We continued this process until we met our sample size requirement of 30 practices, for a total of 55 physicians.
After we obtained informed consent from the physicians and staff, all adult patients (n = 51,078) from the practices were sent a letter by their physician inviting them to participate in the project. After giving informed consent, 5,218 patients participated in a telephone interview during which each patient responded to a survey questionnaire that elicited information on age, sex, race/ethnicity, marital status, smoking status, physical activity, height, weight, daily serving of fruits and vegetables, education level, income level, and type of medical insurance coverage. Of these participants, 4,195 had their medical records abstracted, for a minimum of 20 charts and a maximum of 120 charts per physician. The physicians’ information was obtained by completing a questionnaire and by public data from the state medical licensing board. Practice data were obtained from the physician’s and office manager’s questionnaire responses.
Outcome Assessment (Chart Audits) and Data Collection
We collected the patients’ medical history, history of CVD, data on lipid disorder, hypertension, weight, diabetes mellitus, and smoking management from the medical records. The chart audit process is described in detail in Supplemental Appendix 1, available at http://www.annfammed.org/cgi/content/full/8/1/25/DC1. CVD risk factor management was based upon the medical record from the previous 5 years (1999–2004) for lipid management, and the previous 2 years for blood pressure, diabetes mellitus, smoking cessation, and obesity management.
Measurements and Categorization
Patients were categorized into 4 categories of coronary heart disease risk using the ATP III guideline risk categorization definitions.2 (1) low, (2) moderate, (3) high-risk, and (4) extremely high risk or equivalent of coronary heart disease. These definitions are described in detail in Supplemental Appendix 2, available at http://www.annfammed.org/cgi/content/full/8/1/25/DC1. Patients with a lipid disorder, diabetes mellitus, or hypertension were identified either by documentation of diagnosis in the medical record or prescription of lipid, blood pressure, or glucose-lowering medications with appropriate indications. Low-density lipoprotein (LDL) cholesterol goals were defined using the conservative LDL levels consistent with both the ATP III 2001 and 2004 updated guidelines, because chart audits were based upon data from 1999–2004, at which point the 2001 ATP III standards of care were operative. The LDL goals used were <100 mg/dL for extremely high-risk patients or patients who had the equivalent of coronary heart disease, <130 mg/ dL for moderate- and high-risk patients, and <160 mg/ dL for low-risk patients. Among diabetic patients, an LDL goal was defined as <100 mg/dL, controlled blood pressure as <130/85 mm Hg, and controlled diabetes as glycated hemoglobin A1C (HgbA1C ) levels <7%.7 For patients with hypertension, controlled blood pressure was defined as blood pressure <140/90 mm Hg according to Joint National Committee recommendations (JNC 7).3 Physical activity advice was defined as documentation of physical activity recommendations in the chart. Dietary recommendations were defined as either physician dietary recommendations or documentation of nutrition referrals. CVD risk factor management was defined as management of a lipid disorder, weight control, hypertension control, diabetes control, and smoking cessation. Detailed definitions of CVD risk factor management are described in Supplemental Appendix 3, available at http://www.annfammed.org/cgi/content/full/8/1/25/DC1.
The primary variables of interest for this study were the sex of the patient and physician and their interaction. Patient sex was obtained from the telephone survey and physician sex was obtained from the physician’s questionnaire. Patient variables included age, body mass index, marital status, education, medical insurance, CVD risk category, and medication use. Physician variables included sex, specialty, medical school graduation, number of patients seen per day, hours per week in patient care, and years in the practice. The cut-points were chosen based upon the medians for the number of patients seen per day, hours spent in patient care per week, and years in practice. These physician variables met the criteria for confounding in our modeling selection process. Practice variables included practice size and practices with or without physician assistants or nurse practitioners that met the same modeling criteria or were believed necessary based upon the sampling strategy used.
Statistical Methods
Differences according to the sex of the patient and physician were examined using t tests for continuous variables, and χ2 for categorical variables. A multilevel regression analysis was performed using generalized, linear mixed models to identify patient and physician differences by sex in CVD risk factor management after controlling for patient, physician, and practice confounding variables using SAS 9.1.3 GLIMMIX procedure (SAS Institute, Cary, North Carolina). The physician- and practice-level covariates were determined based upon being different by sex of the patient or physician and being statistically significant in multilevel modeling at P <.10. Practice-level covariates could have no missing data, could not be associated with physician-level variables, and were statistically significant at P <.10 in multilevel modeling or were part of the practice recruitment sampling strategy. We used P <.10 instead of P <.05 or P <.20 for model building to control for confounding bias and to include important covariates that might be excluded if we used the conservative P <.05, and to avoid overadjustment if we used P <.20.39 We report as statistically significant odds ratios and 95% confidence intervals at P <.05.
RESULTS
The number of patients with data analyzed was 4,195, of whom 40% were men and 60% were women. A comparison of patient characteristics and CVD risk factor by sex is displayed in Table 1⇓. Female patients were less likely to be partnered, to have a higher education, and to have private insurance. In addition, coronary heart disease and stroke were less prevalent among female patients, and they were less likely to have a lipid disorder, hypertension, or diabetes.
Characteristics of Male and Female Patients
A comparison of differences in physician characteristics by sex is displayed in Table 2⇓. Of the 39 male physicians and 16 female physicians that participated in this study, female physicians had a significantly higher percentage of female patients in their practices, whereas male physicians saw more patients per day and older patients. There was a trend toward a difference in patient care hours per week (P = .10) and time behind by the end of the day (P = .10), with female physicians being more behind and spending less time per week in patient care.
Characteristics of 55 Participating Physicians
Cardiovascular risk factor management by sex of the patient and physician unadjusted for patient-, physician-, or practice-level confounders is displayed in Table 3⇓. Because some or most of the sex differences shown in Table 3⇓ might be related to confounding by either patient-, physician-, or practice-level characteristics, we performed a multilevel regression analysis.
Cardiovascular Risk Factors Management by Sex of Patient and Physician
Table 4⇓, stratified by patient and physician sex, displays the odds ratios and confidence intervals of these adjusted results. Many of the associations found in Table 3⇑ were lost once adjusted for confounding. By comparing male patients with female patients, we did find that for diabetes care, more male patients were on medications for glucose control, were on angiotensin-converting enzyme (ACE) inhibitor therapy, and were on aspirin prophylaxis. For many CVD risk factors, women physicians were more likely than male physicians to counsel patients regarding diet, weight loss, and physical activity.
Patient and Physician Sex Differences in Cardiovascular Risk Factor Management Using Multiple Level Regression Models
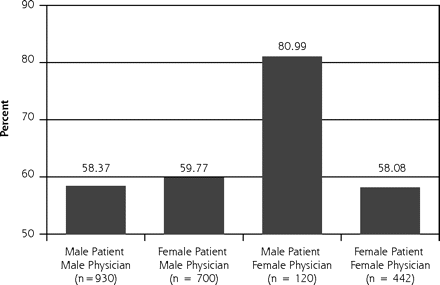
In our multilevel analysis, we found 3 significant patient-physician interactions by sex groupings (Figures 1⇓ through 3⇓⇓). Figure 1⇓ shows that female physicians (P = .03) were more likely to provide diet advice to their female patients with a lipid disorder. Figure 2⇓ shows that female physicians (P = .03) were less likely to prescribe ACE inhibitors to their diabetic female patients. Figure 3⇓ shows that female physicians (P <.05) were less likely to have blood pressure under control in their diabetic female patients.
Lipid management: percentage of patients given diet and weight loss advice.
Diabetes management: percentage of patients on angiotensin-converting enzyme therapy.
Diabetes management: percentage of patients with blood pressure under control.
DISCUSSION
The results of this study are intriguing and show that the quality of care as measured by patients meeting CVD risk factors treatment goals are similar regardless of the sex of the patient or physician. Many CVD risk factor management differences based upon the patient sex as enumerated in Table 3⇑ are lost in multilevel adjustment. There do appear to be some differences in the use of medications regarding diabetes management, with male patients being more likely than female patients to have received glucose-lowering medications, ACE inhibitor therapy, and aspirin therapy. This finding may be due to sex-based differences in the stage and severity of the diabetes or sex-based differences in prescribing behavior. Our findings support previous studies showing that compared with female patients, more male patients get aspirin prophylaxis and ACE inhibitor therapy,40–46 whereas other studies do not confirm this disparity.35,36 The underuse of ACE inhibitors among female patients by female physicians might be explained by ACE inhibitor-associated cough, which has been reported to occur 2 to 3 times more frequently in women than in men.47 We can speculate that the sex concordance of female physicians with their female patients might make them more aware of this adverse effect and therefore prescribe it to fewer women.44
Compared with male physicians, female physicians appear to provide more counseling for a variety of CVD risk factors, including hypertension, obesity, and diabetes. Several other studies have examined male-female differences in preventive services counseling for patients visiting primary care clinics and have found conflicting results.32 A recent analysis showed that among all visitors to primary care practices, patients of female physicians are more likely to receive health behavior counseling with no physician-patient sex concordance.34 Our study showed this same trend among patients with regard to CVD risk factor management. Thus, both male and female patients appear to be more likely to receive counseling services if they have a female physician, or it may be that female physicians are more likely to document these recommendations.
This study has several limitations that should be considered when interpreting our results. We are reporting CVD management behaviors documented in the medical record, and behaviors not documented were considered not done. Previous studies have shown that some behaviors may be performed but are not documented. It is unlikely that the documentation will be different by patient sex; however, documentation might be different by physician sex, because in our study, women physicians were more behind at the end of day and saw fewer patients. We did adjust for these confounding differences in our multilevel regression analysis. In addition, Flocke and Gilchrist,34 using direct observation rather than chart audits, found that female physicians provided more counseling than male physicians, a finding suggesting documentation is less likely to be an issue.
The data represent patients and physicians from southeast New England. The patients who agreed to participate in the study might not be representative of the entire patient population but may represent individuals with interest in CVD risk factor reduction. In a subsample of practices (n = 4), we compared random de-identified chart audits (n = 350) with our identified chart audits (n = 236). The 2 samples were similar in age, marital status, race, prescription benefits, frequency of hypertension, diabetes, and lipid disorders, but there were fewer smokers, more patients in the high-risk and low-risk risk categories for coronary heart disease, and similar percentages of extremely high-risk and fewer moderate-risk patients using the identified chart audits. Thus despite the 10% response rate, our results likely represent the patient characteristics and physician practice patterns of primary care physicians in southeastern New England. Generalizing the results to other patient populations and other physicians must therefore be done with caution. Regarding differences in CVD risk factor management by physician or patient sex, however, it is unlikely the differences in sampling strategy would affect our sex-specific results, because we were focusing on physician behavior as it relates to patient sex.
In summary, the differences in CVD risk factor management by patient and physician sex, after adjusting for patient, physicians, and practice differences, are modest, with the stylistic differences by physician sex in counseling frequency vs medication use having little effect on the frequency of patients meeting CVD guideline-specific goals during a 2- to 5-year period. Future research should explore whether the stylistic differences in CVD risk factor management found in our study have any long-term impact on clinically relevant outcomes, such as myocardial infarctions, strokes, heart failure, and death in longitudinal studies of primary care practice.
Acknowledgments
We would like to thank the following physicians and their practice staff: Charles Cronin III, DO, and Richard DelSesto, MD, E Greenwich, Rhode Island; Daniel Hochberger, MD, Rumford, RI; Robert Cicchelli, MD, and David Cunningham, MD, Middletown, RI; Amrut Patel, MD, Cranston, RI; Linda A. DeLuca, MD, Lincoln, RI; Kim J. Crawford, MD, N Scituate, RI; Robert Lambe, MD, Margaret Koehm, MD, James Macek, MD, and Christine Robb, MD, Plainville, Massachusetts; David Leibowitz, DO, Warren, RI; Colleen A. Cleary, MD, Pawtucket, RI; David Carter, MD, Mark Braun, MD, Peter Hollmann, MD, and Usha Panneerselvam, MD, Cranston, RI; David Kerzer, DO, and Matthew Salisbury, MD, Cranston, RI; Daniel T. Shreve, MD, E Providence, RI; Kelly A. McGarry, MD, Anne Moulton, MD, Iris Tong, MD, and Michelle Stozek, MD, Providence, RI; Leilani Nixon, MD, Putnam, Connecticut; Michael C. Souza, DO, E Providence, RI; David Gorelick, MD, Jayanthi Parameswaran, MD, and Harold Sanders, MD, Newport, RI; Elisabeth Farnum, MD, Cumberland RI; Altaf Girach, MD, and Avanish Mehta, MD, Woonsocket, RI; Robert J. Robbio, MD and John L. Bossian, DO, Wakefield, RI; Bradford Kney, MD, Fall River, MA; Joseph A. DiLorenzo, MD, Cranston, RI; Steven Flood, MD, Richard Popovic, MD, and Patricia Kearney, MD, Foxboro, MA; Hao Yuan Huang, MD, Cranston, RI; Hugo Yamada, MD, and Hua Chung Lu, MD, Lincoln, RI; Michael Baaklini, MD, and Raffi Calikyan, MD, Bristol, RI; Eaine B. Fain, MD, Pawticket, RI; Martin Miner, MD, James Lippencott, MD, Mark Ringiewicz, MD, Priscilla Shube, MD, Hugh Woolverton, MD, and Jeffrey Syme, MD, Swansea, MA; Margaret Sun, MD and James Schwartz, MD, E Providence, RI; Martin Kerzer, DO, Warwick, RI; Jerome McMurray, MA; Mary Roberts, MS; Susan Howard; Barry Clarke; and Sherri Campbell.
Footnotes
-
Conflicts of interest: none reported
-
Funding support: This study was partially funded by Translating ATP III Cholesterol Guidelines into Primary Care Practice (grant No. 1 RO1 HL70804) and Translating ATP III Cholesterol Guidelines Supplement Project–Qualitative Study (grant No. 1 RO1 HL70804).
- Received for publication December 16, 2008.
- Revision received May 21, 2009.
- Accepted for publication June 1, 2009.
- © 2010 Annals of Family Medicine, Inc.