Abstract
PURPOSE We aimed to assess participant-reported factors associated with non–follow-up with colonoscopy in colorectal cancer (CRC) screening.
METHODS In May 2019, we distributed a nationwide cross-sectional questionnaire (n = 4,009) to participants in the Dutch CRC screening program who received a positive fecal immunochemical test (FIT). Among respondents who reported no colonoscopy, we assessed the presence of a contraindication, and those without were compared with those who reported colonoscopy by logistic regression analysis.
RESULTS Of 2,225 respondents (56% response rate), 730 (33%) reported no colonoscopy. A contraindication was reported by 55% (n = 404). Decisional difficulties (odds ratio [OR] = 0.29; 95% CI, 0.18-0.47), lacking the opportunity to discuss the FIT outcome (OR = 0.45; 95% CI, 0.28-0.72), and a low estimated risk of CRC (OR = 0.45; 95% CI, 0.26-0.76) were negatively associated with follow-up. Knowledge items negatively associated with follow-up included having an alternative explanation for the positive FIT (OR = 0.3; 95% CI, 0.21-0.43), having trust in the ability to self-detect CRC (OR = 0.42; 95% CI, 0.27-0.65), and thinking that polyp removal is ineffective (OR = 0.59; 95% CI, 0.43-0.82). The belief that the family physician would support colonoscopy showed the strongest positive association with follow-up (OR = 2.84; 95% CI, 2.01-4.02)
CONCLUSIONS Because decisional difficulties and certain convictions regarding CRC and screening are associated with non–follow-up, personalized screening counseling might be an intervention worth exploring as a means of improving follow-up in the Dutch CRC screening program. Involving family physicians might also prove beneficial.
- follow-up studies
- mass screening
- colorectal neoplasms
- surveys and questionnaires
- Dutch
- counseling
- physicians, family
INTRODUCTION
Population screening programs for colorectal cancer (CRC) aim to decrease CRC-related mortality and morbidity by detecting localized CRC or its precursor lesions, advanced adenomas (AA).1 In the Netherlands, CRC screening was gradually implemented from 2014 onward, with a participation rate of 71.5% in 2019. In contrast to countries in which colonoscopy is the preferred screening approach (eg, the United States), CRC screening in the Netherlands is a 2-step process (Figure 1). First, individuals aged 55-75 years receive a fecal immunochemical test (FIT) test kit and information letter at home. Next, participants with a positive (unfavorable) FIT receive a prescheduled appointment for colonoscopy consultation and are expected to make an autonomous informed decision regarding further participation.2-6 The positive predictive value of the FIT was 6.4% for CRC and 35% for AA in 2018,7 implying that a positive FIT has a greater positive predictive value for advanced lesions in the general population (ie, 41.4%) than any other isolated clinical sign or symptom.8 In 2018, 12.2% (n = 8,654) of FIT-positive participants did not undergo a colonoscopy for unknown reasons.7 That percentage corresponds with those of other European screening programs with similar strategies and compliance rates such as in Spain and Slovenia.9 Non–follow-up with colonoscopy after a positive FIT ranges from 8% in Italy to 47% in the United States, making it an international issue.1,10
Dutch CRC screening protocol.
CRC = colorectal cancer; FIT = fecal immunochemical test.
Because participants who do not follow-up with colonoscopy might comprise a hard-to-reach population, owing to their nonadherence to medical advice, little is known about reasons for non–follow-up with colonoscopy advice on participants’ personal levels.11,12 Existing knowledge mainly originates from retrospective database analysis and, to a lesser extent, cross-sectional surveys.12 Such studies have classified factors associated with non–follow-up with colonoscopy according to participant, provider, and system levels.13,14 On the participant level, these include expecting discomfort of the colonoscopy procedure, fear of cancer detection, poor health behavior, other health issues, and a belief that the screening test result was false positive. On the provider level, scheduling issues negatively influence follow-up, whereas involvement of a clinician and a positive belief in the screening process of the clinician positively influence follow-up. On the system level, follow-up is positively influenced by adequate colonoscopy capacity, use of adequate patient identification tracking systems, performance feedback to primary care, and funding for diagnostic testing.12,15-22
In addition to the above, we identified several additional themes that might play a role in a recent qualitative study exploring reasons for non–follow-up with colonoscopy in the Dutch CRC screening program.23 These include knowledge gaps, distrust of the screening system, low perception of risk, and a preference for personalized screening counseling. In the present study, we incorporated these themes into a questionnaire, with the aim of quantifying the results. We focused on domains with potential modifiable consequences, (ie, knowledge about CRC and screening, participant-associated factors, provider-associated factors, and health system factors to allow us to identify potential targets for intervention).13,14,24 Whereas our research took place in the context of the 2-step Dutch FIT screening program, the above-described barriers to undergoing colonoscopy have also been reported in studies investigating 1-step colonoscopy screening participation in the United States, particularly among disadvantaged subgroups.25-27 Therefore, we expected our results to be applicable to screening programs in the United States and beyond.
METHODS
A questionnaire (Supplemental Table 1) was developed by the research team and external advisors (see Acknowledgments) on the basis of results of our previous qualitative study,23 existing validated questionnaires, and literature on nonparticipation in cancer screening. Questions referred to specific topics (eg, self-efficacy, locus of control). We performed pilot testing of the questionnaire by conducting cognitive interviews with CRC screening participants (n = 5) to ensure relevance and comprehensibility.28,29
Patient Selection
The Dutch screening organization (National Institute for Public Health and the Environment) randomly selected eligible individuals (positive FIT in prior 4-6 months) from their database ScreenIT in May 2019. Of the 15,010 individuals with a positive FIT, those who did not undergo colonoscopy (n = 2,509) were matched with a random sample of those who did (n = 1,500 of 12,501) for age, sex, and prior participation in the screening program. Questionnaires were distributed by postal mail, accompanied by information explaining the aim of the study and a link to an online version (Dutch or English). A postal reminder was sent after 2 weeks.
A reported CRC diagnosis was an exclusion criterion. Questionnaires that reported no colonoscopy were examined for the presence of medical contraindications. The research team decided on categorization of indications on the basis of definitions used by the screening organization.30
Ethics, Consent, and Data Availability
This project was granted a waiver by the medical ethics committee of the Amsterdam UMC (location AMC), reference W19_120#19.153. Consent to participate followed from returning the questionnaire. Questionnaires did not contain questions on personal data that would be traceable to individuals. The data for this study will be shared on reasonable request to the corresponding author.
Statistical Analysis
We performed 2-tailed independent t tests to compare demographic characteristics of respondents who reported a contraindication and no colonoscopy (CIC group) to those who reported no colonoscopy and no contraindication (NC group) and those who reported a colonoscopy (C group). For the latter groups, we used χ2 tests (Spearman) to identify and merge correlated variables, and the association between CRC knowledge and education was examined to identify whether they were collinear. Variables with >2 categories were dichotomized. After univariate analyses for the NC and C groups, we selected variables with the most potential for future intervention (included if P < .2) and (nonmodifiable) sociodemographic covariates that the model needed to adjust for (highlighted in gray in Supplemental Table 2). Missing data analysis of these items suggested that missing data were likely to be missing at random. Because the application of listwise deletion of missing-at-random data might lead to a leftover data sample that might not be representative of the total population, we used multiple imputation by chained equation to estimate missing data points.31,32 All analyses were performed using IBM SPSS Statistics, version 26 (IBM Corp).
RESULTS
Response
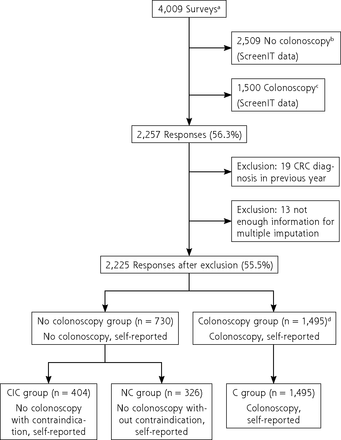
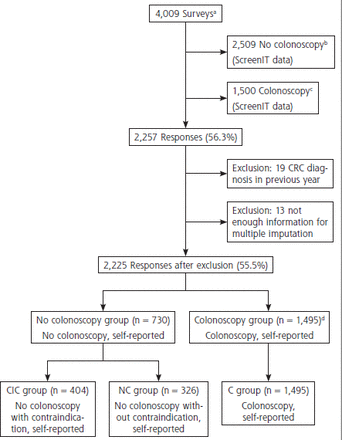
The questionnaire yielded 2,257 responses (of 4,009 individuals in the matched groups) (response rate 56.3%, Figure 2). We excluded 32 respondents for reporting a CRC diagnosis (n = 19) or having too much missing data for multiple imputation (n = 13). Of 2,225 remaining respondents, 1,495 (67.2%) reported a colonoscopy (C group), and 730 (32.8%) did not. In the latter group, 404 respondents reported a contraindication for colonoscopy (CIC group), and 326 did not report a contraindication (NC group).
Overview of inclusion.
C = colonoscopy; CIC = contraindication and no colonoscopy; CRC = colorectal cancer; FIT = fecal immunochemical test; NC = no colonoscopy and no contraindication; ScreenIT = database of the Netherlands National Institute for Public Health and the Environment, which is the Dutch screening organization.
a Participants in the Dutch CRC screening program with a positive FIT 4-6 months ago at time of postal mailing of questionnaires.
b All FIT-positive participants with a positive FIT 4-6 months ago who did not undergo colonoscopy in the Netherlands were sent the questionnaire.
c Control group that did undergo colonoscopy matched to no colonoscopy group for age, sex, and previous participation in the screening program.
d Because it was unlikely that 1,495 individuals responded of the 1,500 that were sent the questionnaire (see C), it appeared that a proportion of individuals who were registered as no colonoscopy (see B) did report a colonoscopy and therefore ended up in the colonoscopy group (see C) in this study.
Demographic Characteristics
Respondents in the CIC group were older than those in the C group and less often had vocational training (Table 1). Compared with the NC group, respondents in the CIC group more often lived with a partner and had ≥1 child, and less often reported not having sufficient financial resources. The respondents in the NC and C groups were comparable for age and sex. The C group more often lived with a partner, had ≥1 child, and lived in a nonurban area. Almost all NC- and C-group respondents were born in the Netherlands (n = 1,622; 92.8% [valid percentage]). Of these respondents, 34% had ≥1 symptom that might be associated with CRC at the time of FIT participation. Having any symptom was not associated with follow-up. The number of NC-group participants who showed up for the intake but did not undergo colonoscopy was 86 (26.4%).
Demographic Characteristics of CIC Group vs NC and C Groups
Missing Data and Variable Selection
Data were missing for 2% to 19.1% of respondents (Supplemental Table 2). To prevent overfitting of the statistical model, we decreased the number of variables.33 We included variables on the basis of the potential for intervention as determined by consensus of the research team (eg, having other medical issues was deemed less appropriate to target in an intervention than having certain convictions regarding CRC and the colonoscopy procedure, and because only 6 respondents indicated having a high estimated probability of CRC after FIT in the NC-group, we decided to include only the low estimated probability score) as well as P value. This led to the imputation and inclusion of 43 variables in the multivariate logistic regression analysis (Supplemental Table 2). Three items measuring trust (trust in test, screening organization, and colonoscopy center) were correlated and therefore merged as a sum score in 1 variable measuring trust (Cronbach α 0.836).
Reasons for Non–Follow-Up in CRC Screening
Medical Contraindication
The majority of participants who did not undergo colonoscopy in this study described a medical contraindication for the procedure (55.3%). Most reported were a colonoscopy in the recent past, having intestinal disease that required regular visits to a medical specialist, and having received advice against colonoscopy from a medical specialist (Table 2).
Reasons for Non–Follow-Up With Colonoscopy
Knowledge About CRC and Screening
Knowledge questions on screening, development of CRC, and colonoscopy procedure were answered incorrectly by 19% to 52% of respondents in the C group (n = 1,495) and 31% to 69% of respondents in the NC group (n = 326) (Table 3). Knowledge questions all had significant but low negative associations with education level (Rs −0.057 to −0.148; P < .05). All knowledge questions, with the exception of 1 on the interpretation of a negative FIT, were answered significantly more often incorrectly in the NC group.
Demographic Characteristics and Descriptive Statistics of CRC Knowledge Items for C and NC Groups
In the multivariate model, knowledge-associated factors negatively associated with follow-up were having an alternative explanation for blood in the stool sample (odds ratio [OR] = 0.3; 95% CI, 0.21-0.43), having trust in the ability to self-detect CRC (OR = 0.42; 95% CI, 0.27-0.65), belief that colonoscopy has a high risk of complications (OR = 0.5; 95% CI, 0.35-0.71), and belief that removal of polyps is not effective in preventing CRC (OR = 0.59; 95% CI, 0.43-0.82) (Table 4). We found no independent association between follow-up and thinking that CRC can be cured if detected early, thinking that 75% of people with CRC die of it, or thinking that CRC always causes symptoms.
Results of Logistic Regression Analysis for NC and C Groups
Participant-Associated Factors
Participant-associated factors negatively associated with follow-up with colonoscopy were receiving negative colonoscopy advice from anyone outside the medical setting (OR = 0.28; 95% CI, 0.13-0.62), having difficulty deciding to undergo colonoscopy (OR = 0.29; 95% CI, 0.18-0.47), low estimation of personal risk for CRC (OR = 0.45; 95% CI, 0.26-0.76), and knowing people with a negative colonoscopy experience (OR = 0.5; 95% CI, 0.31-0.81) (Table 4). Worry about CRC (OR = 1.1; 95% CI, 1.02-1.19) and receiving advice to undergo colonoscopy from anyone (OR = 1.77; 95% CI, 1.22-2.56) were positively associated with follow-up. Having a negative colonoscopy experience compared with having no experience or a positive experience (OR = 1.98; 95% CI, 1.23-3.21) was not negatively associated with follow-up. We found no independent association between follow-up and self-efficacy, locus of control, or fatalism.
Provider-Associated Factors
On the provider level, we found 1 factor associated with follow-up with colonoscopy; this was thinking that the family physician (FP) would be supportive of the procedure (OR = 2.84; 95% CI, 2.01-4.02) (Table 4). However, we found no statistically significant independent association between follow-up and having had contact with the FP to discuss the FIT result.
Health System Factors
On the system level, having little opportunity to discuss the consequences of the positive FIT (OR = 0.45; 95% CI, 0.28-0.72) and being uncomfortable with an unfamiliar colonoscopy location (OR = 0.47; 95% CI, 0.3-0.73) were negatively associated with follow-up (Table 4). Trust in the test, screening organization, and colonoscopy clinic were positively associated with follow-up (OR = 1.17; 95% CI, 1.06-1.31). We found no independent association between follow-up and dislike of the ready-made appointment for intake consultation.
DISCUSSION
Most participants who did not undergo colonoscopy reported a medical contraindication. We found that knowledge of CRC, screening, and colonoscopy was low among the remaining participants in both the NC and C groups. In addition, participants who did not undergo colonoscopy without a contraindication more often held convictions that do not align with current medical knowledge compared with those who underwent colonoscopy. Hearing positive and negative experiences from others influences follow-up in the NC and C groups, and thinking that one’s FP supports follow-up colonoscopy was positively associated with follow-up. Participants who felt they lacked the opportunity to discuss the positive FIT, had difficulty deciding on colonoscopy participation, or were uncomfortable with an unfamiliar colonoscopy location more often did not follow up with colonoscopy.
An unexpected finding was the positive association between follow-up and a negative colonoscopy experience compared with having no experience or a positive experience. Repetition of the logistic regression analysis with a variable measuring any colonoscopy experience compared with no colonoscopy experience (instead of only negative experience) showed a positive association with follow-up (OR = 3.55; 95% CI, 1.95-6.43). Thus, any experience with colonoscopy (negative or positive) is associated with follow-up with colonoscopy. This corroborates studies reporting that prior CRC screening participation is positively associated with future CRC screening participation as well as with diagnostic testing after screening.35-38
Lack of knowledge regarding CRC and CRC screening has been associated with non–follow-up with CRC screening.39,40 A recent study described how misconceptions in these domains are common in multiple European countries.41 However, more information might not be sufficient to improve knowledge because studies report that individuals unwilling to undergo CRC screening tend to avoid evidence-based information on screening,42 and perceptions and beliefs of participants are already formed before they receive the invitation and information about screening.43 In addition, providing additional information on cancer screening programs might not influence the decision to screen yet could increase decisional uncertainty,44 and difficulties in understanding risks combined with preconceptions about screening could lead to a gap in perception between the information provided and participants’ understanding of the information.45 Targeting these misconceptions might therefore need a well–thought-out approach that is specifically tailored to participants’ perceptions.
As we described previously,23 the default invitation and prescheduled appointments used in the Dutch CRC screening program are also used in screening programs for other cancers.46 Such appointments are known to increase follow-up rates.47 Participants might benefit from more assistance in the individual decision-making process that is required in this design. Research has shown that a computer-based decision aid could help with informed decision making for all CRC screening participants including those with low literacy skills.48 However, given that we also found decisional difficulties and lack of opportunity to discuss options to be associated with non–follow-up, an online tool might not be suitable for all participants. Some might prefer not to make an autonomous decision49 or might not feel the need to be completely informed.50 Our finding that respondents who reported that they thought their FP would be supportive of colonoscopy were more likely to undergo colonoscopy appears paradoxical with our finding that an actual visit with the FP did not yield a statistically significant independent association with follow-up with colonoscopy. This could indicate that a personal visit with the FP might not be necessary to improve follow-up, but rather that a reminder from the FP sent to respondents who do not follow-up with colonoscopy might be enough. An international survey of CRC screening programs reported a 12% greater completion rate of colonoscopy when reminders were sent to primary care providers (enabling them to identify and contact FIT-positive patients who did not follow-up with colonoscopy), which would support such an intervention.22 At present, the Dutch program does not provide FPs with the FIT results of their patients.2
A strength of the present study is the design of the questionnaire, which was based on results of an interview study and included pilot testing—both to ensure that questions were relevant. The national scope of the questionnaire increased the generalizability of the results. In addition, we used multiple imputation to correct for missing data, which decreases the probability of biased results compared with deleting missing items listwise.51 The main limitation of the present study was that both compliance with colonoscopy and the presence of medical contraindications were determined based on self-reported data, which is inherent to the method of self-reported questionnaires we had to resort to, owing to privacy legislation that prevented the Dutch screening organization from sharing nonanonymous data. Another limitation is the subjective nature of self-reported data in general, which depends on the quality of the questionnaire and participants’ ability to understand and fill it out. This could have created bias with respect to participants with low literacy levels. In addition, the response rate of participants who did not follow-up with colonoscopy (33%) was relatively low and could have been subject to selection bias as a result. However, because those participants might be considered a hard-to-reach population, owing to their nonadherence to medical advice, research has mainly focused on database exploration. The present study offers insight into personal motives for non–follow-up with CRC screening, which is an area previously understudied. Although we did ask the questions, “Have you ever been diagnosed with cancer (if so, CRC or other)?” and “Were you diagnosed with bowel cancer in the past year?,” we did not have data on the timing and findings of prior colonoscopies. Whereas we asked whether participants were under medical supervision for bowel-related issues, we did not explicitly inquire about family history or personal history of specific high-risk conditions (which are exclusion criteria for participation in CRC screening [Figure 1]). Lastly, some of our findings might be specific for the Dutch setting, in which FIT-positive participants receive a prescheduled appointment for colonoscopy consultation. However, research on colonoscopy screening participation in the United States suggests that most of the factors we detected are applicable to that setting as well, in particular those on the participant and provider levels.25,26,27 At-home FIT screening has increased in popularity in the United States since the start of the COVID-19 pandemic, increasing the relevance of our present results.52
Our results regarding CRC and screening-related knowledge levels suggest that participants might not be well informed in this area. On the participant level, improvement of screening-related knowledge, as well as a focus on more personal guidance and advice, might be an important focus for attention. These issues could be addressed on the system level by considering if information in flyers and during intake consultation could be adjusted to better suit these individual gaps and needs. Given that only 26% of participants in the NC group went to the intake consultation, the intake visit appears to be a less-suitable moment to supply extensive information for those who decide not to have a colonoscopy. Nonetheless, it might be valuable to explore the motives of this specific group of NC-group individuals who did attend the intake visit. On the provider level, involving FPs in the decision-making process could be helpful because their opinion appears to be highly appreciated by participants. Invitations and/or reminders for screening participation could be sent by FPs instead of the screening organization. The added value of FP involvement in increasing colonoscopy follow-up rates has been shown by recent research.22
CONCLUSION
The present study offers comprehensive data on factors associated with non–follow-up with colonoscopy after a positive FIT in the Dutch CRC screening program. We found that lack of knowledge, low risk perception for CRC, and decision-making difficulties are associated with non–follow-up. Improving knowledge levels and offering personal guidance, possibly in the form of personalized screening counseling, as well as the involvement of FPs, might prove beneficial for some FIT-positive individuals.
Acknowledgments
We acknowledge contributions to the development of the questionnaire by Kirsten Douma, Peter Lucassen, Danielle Timmermans, Mechteld Visser, and Mirjam Fransen and contributions to the data analysis by Wim Busschers. We acknowledge the Dutch Screening organization for distribution of the questionnaire, in particular the Foundation of Population Screening East, Foundation of Population Screening Mid-West, Foundation of Population Screening North, Foundation of Population Screening South, and Foundation of Population Screening South-West. In addition, we acknowledge the participants in the Dutch CRC screening program who were willing to cooperate with us in a previous interview study to determine potential barriers to adherence to colonoscopy advice, as well as participants who helped us in the pilot testing of the questionnaire.
Footnotes
Conflicts of interest: authors report none.
Read or post commentaries in response to this article.
Funding support: This study was funded by the Dutch Cancer Society (KWF), grant number UVA 2015-8083. This study was performed independently from the funder with respect to study design; data collection, analysis, and interpretation; writing of the manuscript; and decision to submit the manuscript for publication.
Previous presentation: Preliminary conclusions of this study were presented online to the World Endoscopy Organization CRC SC Expert Working Group on November 20, 2020.
- Received for publication May 1, 2021.
- Revision received June 9, 2022.
- Accepted for publication June 30, 2022.
- © 2022 Annals of Family Medicine, Inc.